Advertisements
Advertisements
Question
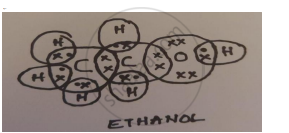
A carbon compound ‘A’ having melting point 156K and boiling point 351K, with molecular formula C2H6O is soluble in water in all proportions.
- Identify ‘A’ and draw its electron dot structure.
- Give the molecular formulae of any two homologues of ‘A’.
Solution
- Ethanol; C2H5OH
- CH3OH and C3H7OH are homologues of ethanol
OR
CH4O and C₃H₈O
APPEARS IN
RELATED QUESTIONS
Write the molecular formula of first two members of homologous series having functional group −Br.
Write the molecular formula of the third member of the homologous series of carbon compounds with general formula CnHO2n+1OH.
The molecular formula of the third member of the homologous series of ketones is:
(a) C4H8O
(b) C3H6O
(c) C5H10O
(d) C6H12O
An organic compound having the molecular formula C3H6O can exist in the form of two isomers A and B having different functional groups. The isomer A is a liquid which is used as a solvent for nail polish. The isomer B is also a liquid. An aqueous solution of one of the lower homologues of B is used for preserving biological specimens in the laboratory
(a) What is compound A?
(b) Write the electron-dot structure of A.
(c) What is compound B?
(d) Write the electron-dot structure of B.
(e) Name the lower homologue of compound B which is used in preserving biological specimens.
Give the dot diagram of the first member of the alcohol.
Give the abbreviated formula of the third member of the alcohol.
 .
.
Distinguish between homologous organs and analogous organs. In which category would you place wings of a bird and wings of a bat? Justify your answer giving a suitable reason.
Complete the following table for the homologous series of alkanes.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methane | CH4 | CH4 | 1 | 1 | -162 |
| Ethane | C2H6 | CH3–CH3 | 2 | 2 | -88.5 |
| Propane | C3H8 | CH3–CH2–CH3 | 3 | 3 | -42 |
| Butane | C4H10 | CH3–CH2–CH2–CH3 | ______ | ______ | 0 |
| Pentane | C5H12 | CH3–CH2–CH2–CH2–CH3 | ______ | ______ | 36 |
| Hexane | C6H14 | CH3–CH2–CH2–CH2–CH2–CH3 | ______ | ______ | 69 |
Complete the following table for homologous series of alcohols.
| Name | Molecular formula | Condensed structural formula | Number of carbon atom | Number of -CH2- units | Boiling point °C |
| Methanol | CH4O | CH3-OH | 1 | 1 | 63 |
| Ethanol | C2H6O | CH3–CH2-OH | 2 | 2 | 78 |
| Propanol | C3H8O | CH3–CH2–CH2-OH | ______ | ______ | 97 |
| Butanol | C4H10O | CH3–CH2–CH2–CH2–OH | ______ | ______ | 118 |
