Advertisements
Advertisements
प्रश्न
Account for the following:
Aniline cannot be prepared by the ammonolysis of chlorobenzene under normal conditions.
उत्तर
In case of chlorobenzene, the C – Cl bond is quite difficult to break as it acquires a partial double bond character due to conjugation. So Under the normal conditions, ammonolysis of chlorobenzene does not yield aniline.
APPEARS IN
संबंधित प्रश्न
Write the reactions of aromatic with nitrous acid.
Explain Hoffmann’s exhaustive alkylation with suitable reactions.
Identify compound 'B' in following series of reactions?
\[\ce{Acetonitrile ->[Na/alcohol] A ->[NaNO2/dil.HCI] B}\]
Which of the following amines cannot be prepared by Gabriel phthalimide synthesis?
Identify product B in the following reaction.
\[\ce{Aniline ->[NaNO2][HCl] A ->[KI] B}\]
Which of the following amines can be prepared by Gabriel synthesis.
(i) Isobutyl amine
(ii) 2-Phenylethylamine
(iii) N-methylbenzylamine
(iv) Aniline
Identify A and B in the following reaction.
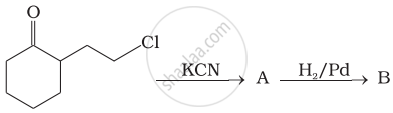
Assertion: Hoffmann’s bromamide reaction is given by primary amines.
Reason: Primary amines are more basic than secondary amines.
Assertion: Aromatic 1° amines can be prepared by Gabriel Phthalimide Synthesis.
Reason: Aryl halides undergo nucleophilic substitution with anion formed by phthalimide.
Identify the compo ds A and B in the following reactions:
\[\ce{A ->[Nitrating mixture] B ->[(i) Sn/cone. HCI][(ii) NaOH] Aniline}\]
