Advertisements
Advertisements
Question
Account for the following:
Aniline cannot be prepared by the ammonolysis of chlorobenzene under normal conditions.
Solution
In case of chlorobenzene, the C – Cl bond is quite difficult to break as it acquires a partial double bond character due to conjugation. So Under the normal conditions, ammonolysis of chlorobenzene does not yield aniline.
APPEARS IN
RELATED QUESTIONS
How do you convert the following: C6H5CONH2 to C6H5NH2
Arrange the following in the increasing order of their pKb values:
C6H5NH2, C2H5NH2, C6H5NHCH3
Give the structures of A, B and C in the following reactions :
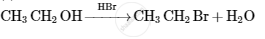
The following amines is the product of Gabriel phthalimide synthesis.
Identify the product obtained, when benzamide is treated with bromine and aqueous sodium hydroxide.
The source of nitrogen in Gabriel synthesis of amines is ______.
Which of the following amines can be prepared by Gabriel synthesis.
(i) Isobutyl amine
(ii) 2-Phenylethylamine
(iii) N-methylbenzylamine
(iv) Aniline
Write following conversions:
nitrobenzene `->` acetanilide
A compound 'A' on reduction with iron scrap and hydrochloric acid gives compound 'B' with molecular formula C6H7N. Compound 'B' on reaction with CHCl3 and alcoholic KOH produces an obnoxious smell of carbylamine due to the formation of 'C'. Identify 'A', 'B' and 'C' and write the chemical reactions involved.
Give reasons for the following:
Ammonolysis of alkyl halides is not a good method to prepare pure primary amines.
