Advertisements
Advertisements
Question
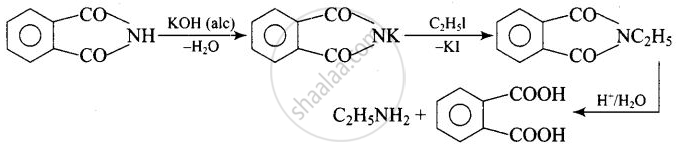
The source of nitrogen in Gabriel synthesis of amines is ______.
Options
Sodium azide, \[\ce{NaN3}\]
Sodium nitrite, \[\ce{NaNO2}\]
Potassium cyanide, \[\ce{KCN}\]
Potassium phthalimide, \[\ce{C6H4(CO)2N– K+}\]
Solution
The source of nitrogen in Gabriel synthesis of amines is Potassium phthalimide, \[\ce{C6H4(CO)2N– K+}\].
Explanation:
Potassium phthalimide is the source of nitrogen in Gabriel’s synthesis.
APPEARS IN
RELATED QUESTIONS
How is ethyl amine prepared from methyl iodide?
Illustrate the following reaction giving suitable example in each case:Gabriel phthalimide synthesis
Answer the following
Identify A and B in the following reactions.
\[\ce{C6H5CH2Br->[alco.][KCN]A ->[Na/ethanol]B.}\]
Answer the following
Explain the ammonolysis of alkyl halides.
____________ can be prepared exclusively by Gabriel phthalimide synthesis.
Among the following amines, the strongest Brönsted base is:
Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product?
(i) Acetyl chloride/pyridine followed by reaction with conc.\[\ce{H2SO4 }\] + conc. \[\ce{HNO3}\].
(ii) Acetic anyhdride/pyridine followed by conc.\[\ce{H2SO4}\] + conc.\[\ce{HNO3}\].
(iii) Dil. HCl followed by reaction with conc.\[\ce{H2SO4}\] + conc.\[\ce{HNO3}\].
(iv) Reaction with conc.\[\ce{HNO3}\] + conc.\[\ce{H2 SO4}\].
Write following conversions:
acetanilide `->` p-nitroaniline
How will you carry out the following conversions?
Assertion: Only a small amount of \[\ce{HCl}\] is required in the reduction of nitro compounds with iron scrap and \[\ce{HCl}\] in the presence of steam.
Reason: \[\ce{FeCl2}\] formed gets hydrolysed to release \[\ce{HCl}\] during the reaction.
