Advertisements
Advertisements
Question
In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one \[\ce{CH2}\] group in the carbon chain, the reagent used as source of nitrogen is ______.
Options
Sodium amide, \[\ce{NaNH2}\]
Sodium azide, \[\ce{NaN3}\]
Potassium cyanide, \[\ce{KCN}\]
Potassium phthalimide, \[\ce{C6H4 (CO)2N– K+}\]
Solution
In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one \[\ce{CH2}\] group in the carbon chain, the reagent used as source of nitrogen is Potassium cyanide, \[\ce{KCN}\].
Explanation:
Potassium cyanide, as cyanide on reduction with sodium metal in alcohol, produces amine with increased carbon atoms.
\[\ce{R - X ->[KCN - KX] R - CN ->[Na/C2H2OH] R - CH2NH2}\]
APPEARS IN
RELATED QUESTIONS
Give the structures of A, B and C in the following reactions :
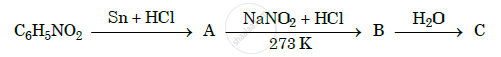
Accomplish the following conversions: Benzamide to toluene
Give the structures of A, B and C in the following reaction:
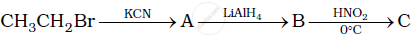
Best method for preparing primary amines from alkyl halides without changing the number of carbon atoms in the chain is ______.
Among the following amines, the strongest Brönsted base is:
The reagents that can be used to convert benzenediazonium chloride to benzene are:
(i) \[\ce{SnCl2/HCl}\]
(ii) \[\ce{CH3CH2OH}\]
(iii) \[\ce{H3PO2}\]
(iv) \[\ce{LiAlH4}\]
How will you carry out the following conversion?
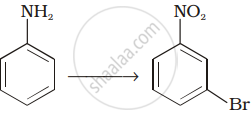
Acetamide and ethyl amide can be distinguished by reacting with.
Which of the following compound gives pink colour on reaction with phthalic anhydride in cone. H2SO4 followed by treatment with NaOH?
Which of the following would not be a good choice for reducing nitrobenzene to aniline?
