Advertisements
Advertisements
प्रश्न
Answer the following question:
How do acids and bases react with each other? What is the name of the process? What product is obtained from these reactions?
उत्तर
i. The acid reacts with a base to form salt and water.
\[\ce{\underset{\text{Acid}}{HA} + \underset{\text{Base}}{BOH} -> \underset{\text{Salt}}{BA} + \underset{\text{water}}{H2O}}\]
ii. It is known that acid generates H+ and base generates OH- ions.
\[\ce{H^{+}_{(aq)} + OH_{(aq)} -> H2O_{(l)}}\]
iii. The H+ ions of acid and OH- ions of a base react with each other to form unionized water. The process is termed as neutralization.
iv. The product obtained out of this reaction is salt and water.
APPEARS IN
संबंधित प्रश्न
Correct and balance the following equation:
N + H  NH3
NH3
What is a chemical equation? Explain with the help of an example.
Balance the following equation and add state symbols:
Zn + HCI → ZnCI2 + H2
Substitute formulae for names and balance the following equation:
Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water and carbon dioxide gas.
With the help of an appropriate example, justify that some of the chemical reactions are determined by Evolution of a gas.
Give chemical equation for the reaction involved in the above case.
State one characteristic of the chemical reaction which takes place when dilute sulphuric acid is added to barium chloride solution.
A metal X forms a salt XSO4. The salt XSO4 forms a clear solution in water which reacts with sodium hydroxide solution to form a blue precipitate Y. Metal X is used in making electric wire and alloys like brass.
(a) What do you think metal X could be?
(b) Write the name, formula and colour of salt XSO4.
(c) What is the blue precipitate Y?
(d) Write a chemical equation of the reaction which takes place when salt XSO4 reacts with sodium hydroxide solution. Give the state symbols of all the reactants and products which occur in the above equation.
Zinc oxide reacts with carbon, on heating, to form zinc metal and carbon monoxide. Write a balanced chemical equation for this reaction. Name (i) oxidising agent, (ii) reducing agent, in this reaction.
Name the type of chemical reaction shown by the following equation:
Fe + CuSO4 → FeSO4 +Cu
Balance the following equation. Also name the product formed.
PbCO3 → PbO +CO2
Complete the following equation:
CH2 + H5OH `("Hot Conc." H_2SO_4)/`>
Balance the equation stepwise.
Ag(s) + HCl(aq) → AgCl ↓+ H2 ↑
Write the chemical equation for the following word equation and balance them.
Calcium + Nitrogen → Calcium nitride
Write the chemical equation for the following word equation and balance them.
Magnesium + Sulphuric acid → Magnesium sulphate + Hydrogen
Write the chemical equation for the following word equation and balance them.
Sodium reacts with water to form sodium hydroxide and hydrogen
Balance the following equation:
PbS + O2 → PbO + SO2
Balance the following equation:
S + H2SO4 → SO2 + H2O
Balance the following equation:
NaHCO3 → Na2CO3 + H2O + CO2
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate moles of salt formed.
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate the mass of salt formed.
Grills of doors and windows are always painted before they are used.
Define: Endothermic reaction
What is thermit process? Where is this process used? Write a balanced chemical equation for the reaction involved.
Write word equation for the following molecular equation:
\[\ce{CuSO4 + 2NaOH -> Na2SO4 + Cu(OH)2↓}\]
Word equation:
State the colour of the products.
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – lead [IV] oxide.
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Light energy
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Electrical energy
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Pressure
Representation of the results of a chemical change – is a chemical equation.
For the equation: FeCl3 + 3NH4OH 3NH4Cl + Fe(OH)3 ↓
Answer the following:
What is the indications of the arrow between the reactants and the products and of the arrow pointing downwards at the end.
Give reason for the following:
Silver salts are kept in dark coloured bottles.
Balance the following simple equation:
N2 + O2 ⇌ NO
Balance the following simple equation:
Al + N2 → AlN
Balance the following simple equation:
Mg + CO2 → MgO + C
Balance the following simple equation:
CaO + HCl → CaCl2 + H2O
Write a balanced equation for the following word equation:
Potassium bromide + Chlorine → Potassium chloride + Bromine
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
C2H5OH + 3O2 → 2CO2 + 2H2O
Writing a chemical reaction in brief by using chemical formulae is called as _______.
Which among the following is(are) double displacement reaction(s)?
- `"Pb" + "CuCl"_2 -> "PbCl"_2 + "Cu"`
- `"Na"_2"SO"_4 + "BaCl"_2 -> "BaSO"_4 + 2"NaCl"`
- `"C" + "O"_2 -> "CO"_2`
- `"CH"_4 + 2"O"_2 -> "CO"_2 + 2"H"_2"O"`
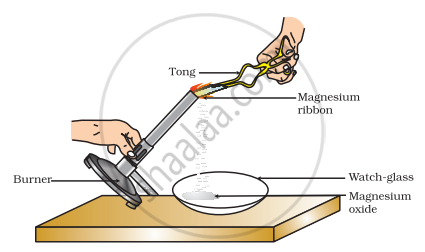
Which of the following is the correct observation of the reaction shown in the above set up?
Dil. HCl is added to Zn granules. How will you prove that chemical change has taken place here? Support your response with two arguments.
