Advertisements
Advertisements
प्रश्न
Assertion: Colloidal solutions show colligative properties.
Reason: Colloidal particles are large in size.
पर्याय
Assertion and reason both are correct and the reason is correct explanation of assertion.
Assertion and reason both are correct but reason does not explain assertion.
Assertion is correct but reason is incorrect.
Both assertion and reason are incorrect.
Assertion is incorrect but reason is correct.
उत्तर
Assertion and reason both are correct but reason does not explain assertion.
Explanation:
Colloidal particles are large in size and hence the number of particle is lesser than the true solution. The lesser number of particles causes lower colligative properties.
APPEARS IN
संबंधित प्रश्न
Give reasons for the following observations: Leather gets hardened after tanning.
Define the following term : Brownian movement
What happens when an emulsion is centrifuged?
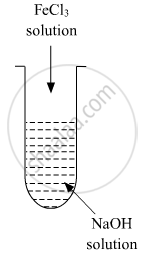
A colloidal sol is prepared by the given method in the figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
Why do we add alum to purify water?
Assertion: An ordinary filter paper impregnated with collodion solution stops the flow of colloidal particles.
Reason: Pore size of the filter paper becomes more than the size of colloidal particle.
Gold number is associated with:-
Define coagulation.
State Hardy-Schulze rule.
6.84 g Al2(SO4)3 is needed to coagulate 2.5 L of As2S3 sol completely in 2.0 hrs. The coagulation value of Al2 (SO4)3 is ______.
