Advertisements
Advertisements
प्रश्न
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
पर्याय
Assertion and reason both are correct and reason is correct explanation of assertion.
Assertion and reason both are wrong statements.
Assertion is correct statement but reason is wrong statement.
Assertion is wrong statement but reason is correct statement.
Assertion and reason both are correct statements but reasson is not correct explanation of assertion.
उत्तर
Assertion is wrong statement but reason is correct statement.
Explanation:
Because of the presence of electron-withdrawing carbonyl group, the alpha hydrogen atom in carbonyl compounds is acidic. In nature, hydrogen is very acidic.
Because the cation is released in the form of \[\ce{H-}\], the anion created after the loss of the -hydrogen atom is resonance stabilised.
APPEARS IN
संबंधित प्रश्न
Write a chemical equation for the following reaction:
Propanone is treated with dilute Ba( OH)2.
Which of the following compounds do not undergo aldol condensation?
(i) \[\ce{CH3 - CHO}\]
(ii) 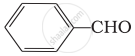
(iii) \[\begin{array}{cc}
\phantom{}\ce{O}\\
\phantom{}||\\
\ce{CH3 - C - CH3}
\end{array}\]
(iv) \[\begin{array}{cc}
\phantom{}\ce{CH3}\\
|\phantom{...}\\
\ce{CH3 - C - CHO}\phantom{..}\\
|\phantom{...}\\
\phantom{}\ce{CH3}\\
\end{array}\]
When liquid ‘A’ is treated with a freshly prepared ammoniacal silver nitrate solution, it gives bright silver mirror. The liquid forms a white crystalline solid on treatment with sodium hydrogensulphite. Liquid ‘B’ also forms a white crystalline solid with sodium hydrogensulphite but it does not give test with ammoniacal silver nitrate. Which of the two liquids is aldehyde? Write the chemical equations of these reactions also.
Which of the following gives aldol con~ensation reaction?
Identify A and B from the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{....................}\\
\phantom{}\ce{2CH3 - C = O ->[Ba(OH)2] A ->[Δ] B + H2O}
\end{array}\]
The major product of the following reaction is:
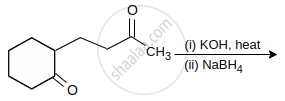
Explain Aldol condensation of ethanal.
Which of the following does not give aldol condensation reaction?
Assertion (A): The final product in Aldol condensation is always α, β-unsaturated carbonyl compound.
Reason (R): α, β-unsaturated carbonyl compounds are stabilised due to conjugation.
What is aldol condensation? Explain it with suitable examples.
