Advertisements
Advertisements
प्रश्न
Identify A and B from the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{....................}\\
\phantom{}\ce{2CH3 - C = O ->[Ba(OH)2] A ->[Δ] B + H2O}
\end{array}\]
उत्तर
\[\begin{array}{cc}
\phantom{..........}\ce{CH3}\phantom{..................}\ce{CH3}\phantom{.......}\ce{CH3}\phantom{....................}\ce{CH3}\phantom{......}\ce{CH3}\phantom{...........}\\
\phantom{..................}|\phantom{.....................}|\phantom{...........}|\phantom{.......................}|\phantom{..........}|\phantom{.....................}\\
\phantom{}\ce{2CH3 - C = O ->[Ba(OH)2] CH3 - C - CH2 - C = O ->[Δ/-H2O]\ce{CH3 - C = CH - C = O + H2O}}\\
\phantom{.............................}|\phantom{.................................}\ce{\underset{(B)}{4-Methypent-3-en-2-one}}\phantom{}\\
\ce{\underset{\underset{(A)}{4-Hydroxy-4-methyl pentan-2-one}}{OH}}\phantom{........................}
\end{array}\]
APPEARS IN
संबंधित प्रश्न
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
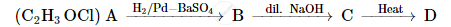
Write the products formed when CH3CHO reacts with the following reagents: CH3CHO in the presence of dilute NaOH
How will you bring about the following conversion?
Ethanal to but-2-enal
How will you convert ethanal into the following compound?
Butane-1, 3-diol
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
How will you bring about the following conversion in not more than two steps?
Benzaldehyde to 3-Phenylpropan-1-ol
Describe the following:
Cross aldol condensation
Complete the synthesis by giving missing starting material, reagent or product.
\[\begin{array}{cc}
\ce{C6H5CHO}\phantom{............}\\
\phantom{........}\ce{+\phantom{......}\ce{->[dil.NaOH][\Delta]}}\phantom{...}\\
\ce{CH3CH2CHO}\phantom{............}
\end{array}\]
Give reasons Acetylation of aniline reduces its activation effect.
Why is alpha (α) hydrogen of carbonyl compounds acidic in nature?
Write chemical equations of the following reaction :
Propanone is treated with dilute Ba (OH)2-.
Write a chemical equation for the following reaction:
Propanone is treated with dilute Ba( OH)2.
What is substituted imine called?
Explain aldol condensation reaction in detail.
Which of the following compounds do not undergo aldol condensation?
(i) \[\ce{CH3 - CHO}\]
(ii) 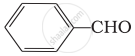
(iii) \[\begin{array}{cc}
\phantom{}\ce{O}\\
\phantom{}||\\
\ce{CH3 - C - CH3}
\end{array}\]
(iv) \[\begin{array}{cc}
\phantom{}\ce{CH3}\\
|\phantom{...}\\
\ce{CH3 - C - CHO}\phantom{..}\\
|\phantom{...}\\
\phantom{}\ce{CH3}\\
\end{array}\]
Why are carboxylic acids more acidic than alcohols or phenols although all of them have hydrogen atom attached to a oxygen atom \[\ce{(-O-H)}\]?
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
Assertion: Aromatic aldehydes and formaldehyde undergo Cannizaro reaction.
Reason: Aromatic aldehydes are almost as reactive as formaldehyde.
Give reasons to support the answer:
Presence of Alpha hydrogen in aldehydes and ketones is essential for aldol condensation.
Cross aldol condensation occurs between
\[\ce{CH3-CH2-CHO ->[dil][alkali] Product}\]
The product in the above reaction is:
The major product of the following reaction is:
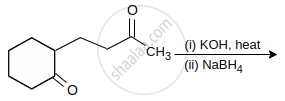
Explain Aldol condensation of ethanal.
Why is the α-hydrogens of aldehydes and ketones are acidic in nature?

Identify A and B:
When acetaldehyde is treated with dilute NaOH, the following reaction is observed.
\[\begin{array}{cc}
\ce{2CH3 - CHO ->[dil.NaOH] CH3 - CH - CH2 - CHO}\\
\phantom{...............}|\\
\phantom{.................}\ce{OH}
\end{array}\]
- What are the functional groups in the product?
- Can another product be formed during the same reaction? (Deduce the answer by doing atomic audit of reactant and product).
- Is this an addition reaction or condensation reaction?
