Advertisements
Advertisements
प्रश्न
Why is alpha (α) hydrogen of carbonyl compounds acidic in nature?
उत्तर
The acidity of α carbonyl group is due to strong electron withdrawing effect of carbonyl group and resonance stabilization of conjugate base.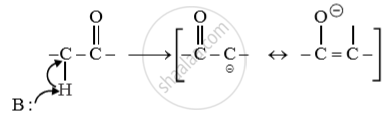
APPEARS IN
संबंधित प्रश्न
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
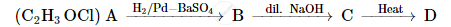
Write the products formed when CH3CHO reacts with the following reagents: CH3CHO in the presence of dilute NaOH
What is substituted imine called?
Cannizaro’s reaction is not given by ______.
Which of the following compounds do not undergo aldol condensation?
(i) \[\ce{CH3 - CHO}\]
(ii) 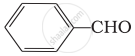
(iii) \[\begin{array}{cc}
\phantom{}\ce{O}\\
\phantom{}||\\
\ce{CH3 - C - CH3}
\end{array}\]
(iv) \[\begin{array}{cc}
\phantom{}\ce{CH3}\\
|\phantom{...}\\
\ce{CH3 - C - CHO}\phantom{..}\\
|\phantom{...}\\
\phantom{}\ce{CH3}\\
\end{array}\]
Which of the following conversions can be carried out by Clemmensen Reduction?
(i) Benzaldehyde into benzyl alcohol
(ii) Cyclohexanone into cyclohexane
(iii) Benzoyl chloride into benzaldehyde
(iv) Benzophenone into diphenyl methane
Explain Aldol condensation of ethanal.
Which of the following does not give aldol condensation reaction?
Why is the α-hydrogens of aldehydes and ketones are acidic in nature?
When acetaldehyde is treated with dilute NaOH, the following reaction is observed.
\[\begin{array}{cc}
\ce{2CH3 - CHO ->[dil.NaOH] CH3 - CH - CH2 - CHO}\\
\phantom{...............}|\\
\phantom{.................}\ce{OH}
\end{array}\]
- What are the functional groups in the product?
- Can another product be formed during the same reaction? (Deduce the answer by doing atomic audit of reactant and product).
- Is this an addition reaction or condensation reaction?
