Advertisements
Advertisements
प्रश्न
Why is alpha (α) hydrogen of carbonyl compounds acidic in nature?
उत्तर
The acidity of α carbonyl group is due to strong electron withdrawing effect of carbonyl group and resonance stabilization of conjugate base.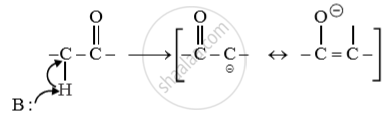
APPEARS IN
संबंधित प्रश्न
How will you convert ethanal into the following compound?
But-2-enal
Write chemical equations of the following reaction :
Benzoyl chloride is hydrogenated in the presence of `"Pd"/(BaSO_4)`
Explain aldol condensation reaction in detail.
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
Give reasons to support the answer:
Presence of Alpha hydrogen in aldehydes and ketones is essential for aldol condensation.
Which of the following gives aldol con~ensation reaction?
Predict the reagent for carrying out the following transformations:
Ethanal to 3-hydroxy butanal
\[\ce{CH3-CH2-CHO ->[dil][alkali] Product}\]
The product in the above reaction is:
The major product of the following reaction is:
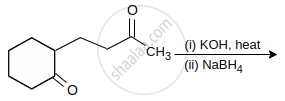
Explain Aldol condensation of ethanal.
