Advertisements
Advertisements
प्रश्न
Explain aldol condensation reaction in detail.
उत्तर
- Aldehydes containing at least one α–hydrogen atom undergo a reaction in presence of dilute alkali (dilute NaOH, KOH, or Na2CO3) as the catalyst to form β-hydroxy aldehydes (aldol). This reaction is known as the aldol reaction.
- Formation of aldol is an addition reaction.
- Aldol formed from aldehyde having α-hydrogens undergoes subsequent elimination of water molecule on warming. This gives rise to α,β-unsaturated aldehyde.
- The overall reaction is called aldol condensation. It is a nucleophilic addition-elimination reaction.
- The reaction can be given as,
\[\begin{array}{cc}
\ce{\underset{\text{Aldehyde}}{2R - CH2 - CHO} ->[aq.NaOH] R - CH2 - CH - CHR - CHO}\\
\phantom{.......................}|\\
\phantom{..........................}\ce{\underset{\text{Aldol}}{OH}}
\end{array}\]
\[\begin{array}{cc}
\ce{R - CH2 - CH - CHR - CHO ->[-H2O][warm] \underset{\text{α,β-Unsaturated aldehyde}}{R - CH = CR - CHO}}\\
|\phantom{.......................................}\\
\ce{\underset{Aldol}{OH}}\phantom{.....................................}
\end{array}\]
e.g.
- Ketones containing at least two α-hydrogen also undergo aldol condensation reaction and give an α,β-unsaturated ketone.
e.g.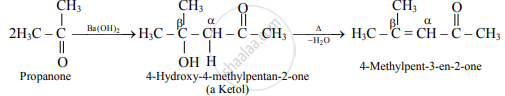
APPEARS IN
संबंधित प्रश्न
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
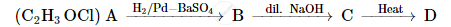
How will you bring about the following conversion?
Ethanal to but-2-enal
What is meant by the following term? Give an example of the reaction in the following case.
Aldol
How will you convert ethanal into the following compound?
But-2-enal
Write structural formulas and names of four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde acts as nucleophile and which as electrophile.
Describe the following:
Cross aldol condensation
Give reasons Acetylation of aniline reduces its activation effect.
Write chemical equations of the following reaction :
Propanone is treated with dilute Ba (OH)2-.
Write a chemical equation for the following reaction:
Propanone is treated with dilute Ba( OH)2.
Compounds A and C in the following reaction are:
\[\ce{CH3CHO ->[(i) CH3MgBr][(ii) H2O] (A) ->[H2SO4, Δ] (B) ->[Hydroboration oxidation] (C)}\]
Which of the following conversions can be carried out by Clemmensen Reduction?
(i) Benzaldehyde into benzyl alcohol
(ii) Cyclohexanone into cyclohexane
(iii) Benzoyl chloride into benzaldehyde
(iv) Benzophenone into diphenyl methane
What product will be formed on reaction of propanal with 2-methylpropanal in the presence of \[\ce{NaOH}\]? What products will be formed? Write the name of the reaction also.
Compound ‘A’ was prepared by oxidation of compound ‘B’ with alkaline \[\ce{KMnO4}\]. Compound ‘A’ on reduction with lithium aluminium hydride gets converted back to compound ‘B’. When compound ‘A’ is heated with compound B in the presence of \[\ce{H2SO4}\] it produces fruity smell of compound C to which family the compounds ‘A’, ‘B’ and ‘C’ belong to?
Why are carboxylic acids more acidic than alcohols or phenols although all of them have hydrogen atom attached to a oxygen atom \[\ce{(-O-H)}\]?
Assertion: The α-hydrogen atom in carbonyl compounds is less acidic.
Reason: The anion formed after the loss of α-hydrogen atom is resonance stabilised.
Give reasons to support the answer:
Presence of Alpha hydrogen in aldehydes and ketones is essential for aldol condensation.
Identify A and B from the following reaction:
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{....................}\\
\phantom{}\ce{2CH3 - C = O ->[Ba(OH)2] A ->[Δ] B + H2O}
\end{array}\]
Convert the following:
Acetaldehyde to But-2-enal
Predict the reagent for carrying out the following transformations:
Ethanal to 3-hydroxy butanal
\[\ce{CH3-CH2-CHO ->[dil][alkali] Product}\]
The product in the above reaction is:
Which of the following does not give aldol condensation reaction?
Which of the following compounds will undergo self-condensation in the presence of dilute NaOH solution?
Why is the α-hydrogens of aldehydes and ketones are acidic in nature?
Assertion (A): The final product in Aldol condensation is always α, β-unsaturated carbonyl compound.
Reason (R): α, β-unsaturated carbonyl compounds are stabilised due to conjugation.

Identify A and B:
