Advertisements
Advertisements
प्रश्न
Balance the following equation:
Zn + HNO3 `→` Zn(NO3)2 + H2O + NO2
उत्तर
Zn + 4HNO3 `→` Zn(NO3)2 + 2H2O + 2NO2
APPEARS IN
संबंधित प्रश्न
Fill in the following blank with suitable word:
A solution made in water is known as an ........... solution and indicated by the symbol ...........
Convey the following information in the form of a balanced chemical equation:
"An aqueous solution of ferrous sulphate reacts with an aqueous solution of sodium hydroxide to form a precipitate of ferrous hydroxide and sodium sulphate remains in solution."
When water is added gradually to a white solid X, a hissing sound is heard and a lot of heat is produced forming a product Y. A suspension of Y in water is applied to the walls of a house during white washing. A clear solution of Y is also used for testing carbon dioxide gas in the laboratory.
(a) What could be solid X? Write its chemical formula.
(b) What could be product Y? Write its chemical formula.
(c) What is the common name of the solution of Y which is used for testing carbon dioxide gas?
(d) Write chemical equation of the reaction which takes place on adding water to slid X.
(e) Which characteristic of chemical reactions is illustrated by this example?
Write the balanced chemical equation of the following reaction.
iron pyrites(FeS2) + oxygen → ferric oxide + sulphur dioxide
Answer the following question:
How do acids and bases react with each other? What is the name of the process? What product is obtained from these reactions?
Write a balanced equation for the following word equation:
Potassium bicarbonate → Potassium carbonate + Water + Carbon dioxide
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
PbO2 +4HCl → PbCl2 + H2O + Cl2
Match the columns.
| Reactants | Products | Types of chemical reaction | ||
| 1. | MgH2 | → | Mg + H2 | Endothermic |
| 2. | 2H2S + SO2 | → | 3S + 2H2O | Oxidation |
| 3. | CaO + H2O | → | Ca(OH)2 + heat | Exothermic |
| Redox | ||||
Which among the following is(are) double displacement reaction(s)?
- `"Pb" + "CuCl"_2 -> "PbCl"_2 + "Cu"`
- `"Na"_2"SO"_4 + "BaCl"_2 -> "BaSO"_4 + 2"NaCl"`
- `"C" + "O"_2 -> "CO"_2`
- `"CH"_4 + 2"O"_2 -> "CO"_2 + 2"H"_2"O"`
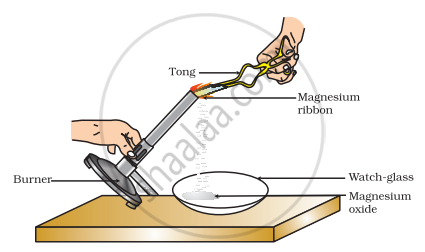
Which of the following is the correct observation of the reaction shown in the above set up?
