Advertisements
Advertisements
प्रश्न
Classify the following metals based on their reactivity.
Cu, Zn, Ca, Mg, Fe, Na, Li, Hg
| More reactive | Moderately reactive | Less reactive |
उत्तर
| More reactive | Moderately reactive | Less reactive |
| Ca | Zn | Cu |
| Na | Mg | Hg |
| Li | Fe |
APPEARS IN
संबंधित प्रश्न
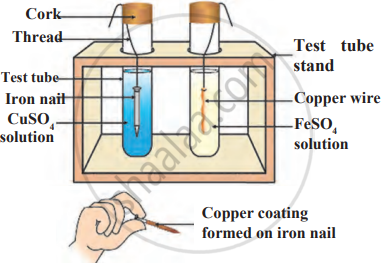
What do you observe when a few pieces of iron are dropped in a blue solution of copper sulphate?
How will you obtain Silver chloride from silver nitrate.
Also give balanced equations for the reactions
What do you observe when Magnesium ribbon is burnt in oxygen.
Write chemical equation for the event.
Iron filings are dropped in aqueous solution of copper sulphate.
Write the chemical equation for the event.
A reaction was brought about between ferric oxide and aluminum.
Divide the metals Cu, Zn, Ca, Mg, Fe, Na, Li into three groups, namely reactive metals, moderately reactive metals and less reactive metals.
State what is meant by the ‘reactivity series of metals’
With reference to Water explain with suitable examples of how the reactivity of the metals could be differentiated.
With reference to Acid explain with a suitable example of how the reactivity of the metals could be differentiated.
Give a balanced equation for the following type of reaction:
A displacement reaction in which a metal above hydrogen in the reactivity series, displaces another metal from the solution of its compound.
Observe the following diagram and identify the type of reaction and write observation.
A metal A, which is used in thermite process, when heated with oxygen gives an oxide B, which is amphoteric in nature. Identify A and B. Write down the reactions of oxide B with HCl and NaOH.
A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black colour product. Identify M and black coloured product and also explain the reaction of M with oxygen.
A solution of CuSO4 was kept in an iron pot. After few days the iron pot was found to have a number of holes in it. Explain the reason in terms of reactivity. Write the equation of the reaction involved.
Of the three metals X, Y and Z. X reacts with cold water, Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in order of increasing reactivity.
