Advertisements
Advertisements
प्रश्न
Complete the following chemical equations:
`(i) Cr_2O_7^(2-)+6Fe^(2+)+14H^+ ->`
`(ii) 2CrO_4^(2-)+2H^+ ->`
`(iii) 2MnO_4^-+5C_2O_4^(2-)+16H^+ ->`
उत्तर
` (i)6Fe^(2+)+Cr_2O_7^(2-)+14H^+ -> 2Cr^(3+)+6Fe^(3+)+7H_2O`
(ii) 2CrO42- + 2H+ → Cr2O72- + H2O
(iii) 2MnO4- + 5C2O42- + 16H+ → 2Mn2+ + 10CO2 + 8H2O
APPEARS IN
संबंधित प्रश्न
Which of the d-block elements may not be regarded as the transition elements?
How is the variability in oxidation states of transition metals different from that of the non-transition metals? Illustrate with examples.
Compare the stability of +2 oxidation state for the elements of the first transition series.
What is meant by 'disproportionation'? Give two examples of disproportionation reaction in aqueous solution.
Why does the density of transition elements increase from Titanium to Copper? (at. no. Ti = 22,
Cu = 29)
Which of the following statements is not correct?
Answer the following question:
Which element of the first transition series has highest second ionisation enthalpy?
On the basis of the figure given below, answer the following questions:
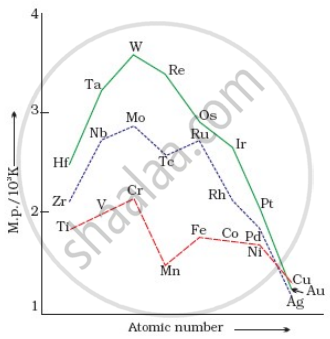
- Why Manganese has lower melting point than Chromium?
- Why do transition metals of 3d series have lower melting points as compared to 4d series?
- In the third transition series, identify and name the metal with the highest melting point.
The compounds of \[\ce{Ti^4+}\] ions are colourless due to ______.
Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following point:
Ionisation enthalpies
