Advertisements
Advertisements
प्रश्न
Consider two elements 'A' (Atomic number 17) and 'B' (Atomic number 19) :
(i) Write the positions of these elements in the modern periodic table giving justification.
(ii) Write the formula of the compound formed when 'A' combines with 'B.'
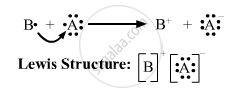
(iii) Draw the electron dot structure of the compound and state the nature of the bond formed between the two elements.
उत्तर
(i) Position of the elements in the periodic table:
| Element | Period | Group |
| A | 3 | 17 |
| B | 4 | 1 |
(ii)
Atomic number of A = 17
Electronic configuration A = 2,8,7
Number of valence electrons of A = 7
Valency of A = 8 - 7 = 1
Atomic number of B = 19
Electronic configuration B = 2,8,8,1
Number of valence electrons of B = 1
Valency of A = 1
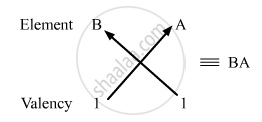
So, the formula of the compound formed when elements A and B combine is BA.
(iii)
APPEARS IN
संबंधित प्रश्न
Which element has a total of two shells, with three electrons in its valence shell?
The atomic radii of three elements X, Y and Z of a period of the periodic table are 186 pm; 104 pm and 143 pm respectively. Giving a reason, arrange these elements in the increasing order of atomic numbers in the period.
In which part of a group would you separately expect the elements to have the greatest metallic character
Which of the following element does not lose an electron easily?
(a) Na
(b) F
(c) Mg
(d) Al
An element A has atomic number 14. To which period does this element belong and how many elements are there in this period.
What will be the valency of an element of atomic number 9 (nine)?
Choose the most appropriate answer from the following list of oxides which fit the description.
A basic oxide.
Formula of ion of A is A2+. Element A probably belongs to ______ group.
Based on the group valency of element write the molecular formula of the following compound giving justification:
Oxide of first group elements.
Name the period which is the shortest period of the periodic table.
