Advertisements
Advertisements
प्रश्न
Draw the electron-dot structure of CO2 compound and state the type of bonding.
उत्तर
The electron-dot structure of CO2 is:
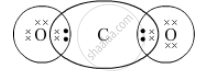
Both oxygen and carbon share their two electrons to form a covalent bond in carbon dioxide, CO2.
APPEARS IN
संबंधित प्रश्न
Explain why carbon forms compounds mainly by covalent bond.
What type of bonds are present in water molecule? Draw the electron-dot structure of water (H2O).
will CCl4 conduct electricity or not?
give reason for your choice.
What type of bonding would you expect between Carbon and Chlorine?
Which of the following has a triple bond as well as single bonds?
(a) ethene
(b) methane
(c) ethyne
(d) nitrogen
The number of isomers formed by the hydrocarbon with molecular formula C5H12 is:
(a) 2
(b) 5
(c) 3
(d) 4
Complete the following:
In case of non-polar covalent bond, the covalent bond is formed in the ______ of atoms and shared electrons are ______ distributed. (corner, middle, equally, unequally)
Give two example in following case:
Liquid non polar compounds
The covalent bond in which the electrons are shared equally between the combining atoms.
Give examples for the following:
Two gaseous non polar compounds.
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
X is likely to have a :
An element L consists of molecules.
What type of bonding is present in the particles that make up L?
Draw an electron dot diagram to show the formation of the following compound.
Magnesium chloride [ H=1, C=6, Mg=12, Cl=17].
Element A has 2 electrons in its M shell. Element B has atomic number 7.
If the compound formed between A and B is melted and an electric current is passed through the molten compound, the element A will be obtained at the ______ and B at the ______ of the electrolytic cell.
Define a covalent bond.
In the formation of electrovalent compounds, electrons are transferred from one element to another. How are electrons involved in the formation of a covalent compound?
Name the anion present in the following compound:
When a barium chloride solution is added to a solution of compound B, a white precipitate insoluble in dilute hydrochloric acid is formed.
Considering MgCl2 as ionic compound and CH4 as covalent compound give any two differences between these two compounds.
Which of the following compound(s) possesses a high melting point?
