Advertisements
Advertisements
प्रश्न
Draw the electron-dot structure of a hydrogen chloride molecule.
उत्तर
The electron-dot structure of a hydrogen chloride molecule is:
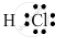
It is formed by sharing one electron by the hydrogen and chlorine atoms.
APPEARS IN
संबंधित प्रश्न
List three characteristic properties of covalent compounds.
An element L consists of molecules.
What type of bonding is present in the particles that make up L?
Fill in the blank in the following sentence:
Two chlorine atoms combine to form a molecule. The bond between them is known as ..........
Fill in the following blank with suitable word:
The general formula CnH2n for cycloalkanes is the same as that of ...........
Explain why, ionic compounds conduct electricity in solution whereas covalent compounds do not conduct electricity.
Describe the structure of graphite with the help of a labelled diagram.
Why is graphite a good conductor of electricity but diamond is a non-conductor of electricity?
The solution of one of the following compounds will not conduct electricity. This compounds is:
(a) NaCl
(b) CCl4
(c) MgCl2
(d) CaCl2
Draw all possile structural formulae of compound from their molecular formula given below.
C3H4
Molecular formula of Propane is C3H8 , write the structural formula of propane.
Explain the following:
Polar covalent compounds are good conductors of electricity.
Choose the correct answer from the options given below
Condition favorable for formation of a covalent bond is
Give reason as to why hydrogen chloride can be termed as a polar covalent compound.
State the type of bond formed when the combining atom has zero E.N. difference.
Name the anion present in the following compound:
When a barium chloride solution is added to a solution of compound B, a white precipitate insoluble in dilute hydrochloric acid is formed.
Write a short note.
Aromatic hydrocarbons
Which of the following statements are usually correct for carbon compounds? These
- are good conductors of electricity
- are poor conductors of electricity
- have strong forces of attraction between their molecules
- do not have strong forces of attraction between their molecules.
Cation is formed when ______.
Assertion (A): Melting point and boiling point of ethanol are lower than that of sodium chloride.
Reason (R): The forces of attraction between the molecules of ionic compounds are very strong.
Carbon can neither form C4- cation nor C4 anion. Why?
