Advertisements
Advertisements
प्रश्न
Give reasons for the following : Boiling point of ethanol is higher in comparison to methoxymethane.
उत्तर
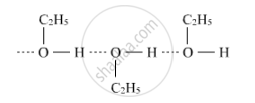
Ethanol forms intermolecular hydrogen bonds due to the presence of hydrogen attached to the electronegative oxygen atom. As a result, ethanol exists as associated molecules while methoxymethane does not. Hence, the boiling point of ethanol is higher than that of methoxymethane.
संबंधित प्रश्न
Give reasons for the following: Butan-1-ol has a higher boiling point than diethyl ether.
Alcohols have high boiling points because of ____________.
Isopropyl alcohol is obtained by reacting which of the following alkenes with concentrated H2SO4 followed by boiling with H2O?
Arrange the following compounds in increasing order of boiling point. Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
Explain why alcohols and ethers of comparable molecular mass have different boiling points?
Write a note on Kolbe's reaction
Convert the following:
acetaldehyde to isopropyl alcohol.
How is the following conversion carried out?
Methyl magnesium bromide → 2-Methylpropan-2-ol.
How is the following conversion carried out?
\[\ce{Methyl magnesium bromide-> 2-Methylpropan-2-ol}\]
How is the following conversions carried out?
\[\ce{Methyl magnesium bromide → 2-Methylpropan-2-ol}\].
