Advertisements
Advertisements
प्रश्न
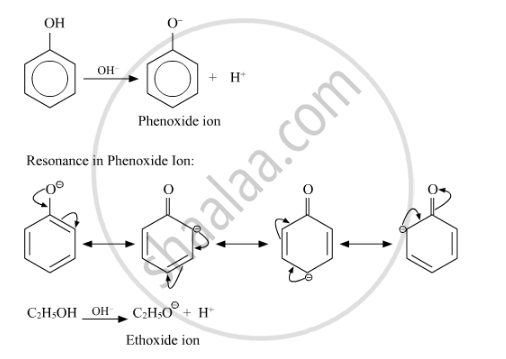
Give reasons for the following : Phenol is more acidic than ethanol.
उत्तर
The phenoxide ion, produced by losing of proton by phenol, is stabilised by resonance due to delocalisation of the negative charge on the benzene ring. Ethoxide ion, however, is not stabilised by resonance. On the other hand, it is further destabilised by positive inductive effect of alkyl group.
संबंधित प्रश्न
Give reasons for the following: Butan-1-ol has a higher boiling point than diethyl ether.
Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Write the structures of A, B and C in the following reactions :
\[ C_6 H_5 {NO}_2 \to^{Sn/HCI} A \to^{{NaNO}_2 /HCI}_{273 K} B \to^{H_2 O}_∆ C\]
Write a chemical reaction to show that the open structure of D-glucose contains the following :
Five alcohol groups
Isopropyl alcohol is obtained by reacting which of the following alkenes with concentrated H2SO4 followed by boiling with H2O?
What is esterifications? How is an ester obtained from alcohol or phenol?
How are the following conversions carried out?
Methyl magnesium bromide → 2 -Methylpropan-2-ol.
How are the following conversions carried out?
\[\ce{Methyl magnesium bromide -> 2-Methylpropan-2-ol}\]
How is the following conversion carried out?
\[\ce{Methyl magnesium bromide-> 2-Methylpropan-2-ol}\]
