Advertisements
Advertisements
प्रश्न
Glucose on reaction with HI gives n-hexane. What does it suggest about the structure of glucose?
उत्तर
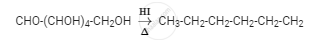
The reaction of glucose with HI giving n-hexane suggests that all the six carbon atoms are linked in a straight chain, as shown in the reaction given below:
APPEARS IN
संबंधित प्रश्न
Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Enlist the properties of glucose that can not be explained on the basis of open chain structure of it
Differentiable between the following:
Amylose and Amylopectin
Choose the appropriate answer(s) for the below representation from the options given
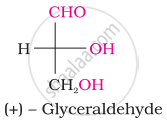
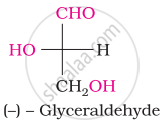
What is the most abundant organic compound on earth?
Glucose does not give Schiff’s test because of the formation of cyclic ____________.
A solution of D-glucose in water rotates the plane polarised light ____________.
On the basis of which evidences D-glucose was assigned the following structure?
\[\begin{array}{cc}
\ce{CHO}\\
|\phantom{....}\\
\phantom{..}\ce{(CHOH)4}\\
|\phantom{....}\\
\phantom{..}\ce{CH2OH}
\end{array}\]
Account for the following:
What happens when D – glucose is treated with the following reagents
HNO3
Consider the following reactions:
(i) \[\ce{Glucose + R-OH ->[Conc. HNO3] [A] ->[X eq of][(CH3CO)2O] Acetyl derivative}\]
(ii) \[\ce{Glucose ->[Ni/H2] [A] ->[Y eq of][(CH3CO)2O] Acetyl derivative}\]
(iii) \[\ce{Glucose ->[Z eq of][(CH3CO)2O] Acetyl derivative}\]
'X, 'Y' and 'Z' in these reactions are respectively:
