Advertisements
Advertisements
प्रश्न
How are amines classified?
उत्तर
Classification of Amines:
i. Amines are classified as primary (1°), secondary (2°), and tertiary (3°) amines.
ii. Their structures are obtained in a simple way by replacing one, two, or three hydrogen atoms of NH3 molecule by alkyl/aryl groups.
iii. Secondary and tertiary amines are further classified as simple or symmetrical amines and mixed or unsymmetrical amines.
iv. When all the alkyl or aryl groups on nitrogen are the same, it is a simple amine. If these groups are different, then the amine is a mixed amine.
v. Amines are also divided into two major classes, namely, aliphatic and aromatic amines on the basis of the nature of the groups attached to the nitrogen atom.
APPEARS IN
संबंधित प्रश्न
Classify the following amine as primary, secondary or tertiary:
(C2H5)2NH
In the following
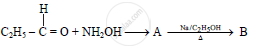
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
Choose the most correct option.
The hybridisation of nitrogen in primary amine is ____________.
Isobutylamine is an example of ______.
Choose the most correct option.
Which type of amine does produce N2 when treated with HNO2?
Choose the most correct option.
Carbylamine test is given by ____________.
Choose the most correct option.
Identify ‘B’ in the following reactions
\[\ce{CH3 - C ≡ N ->[Na/C2H5OH] A ->[NaNO2/dilHCl]B}\]
What are amines?
How are amines classified depending on the functional group? Give one example of each class of amines.
Which of the following amines is most basic in nature in aqueous phase?
Assertion: Acetamide on reaction with KOH and bromine gives acetic acid.
Reason: Bromine catalyses hydrolysis of acetamide.
\[\ce{Aniline + benzoylchloride ->[NaOH] C6H5 - NH - COC6H5}\] this reaction is known as ____________.
The product formed by the reaction an aldehyde with a primary amine ____________.
When aniline reacts with acetic anhydride the product formed is ____________.
The order of basic strength for methyl substituted amines in aqueous solution is ____________.
\[\ce{C6H5NO2 ->[Fe/HCl] A ->[NaNO2/HCl][273 K] B ->[H2O][283 K] C}\] ‘C’ is:
IUPAC name for the amine is:
\[\begin{array}{cc}
\phantom{.}\ce{CH3}\\
|\phantom{..}\\
\ce{CH3 - N - C - CH2 - CH3}\\
\phantom{.}|\phantom{.....}|\phantom{........}\\
\phantom{}\ce{CH3}\phantom{..}\ce{C2H5}\phantom{....}
\end{array}\]
Write a short note on the following.
Ammonolysis
Write a short note on the following:
Gabriel phthalimide synthesis
Write a short note on the following.
Mustard oil reaction
How will you distinguish between primary secondary and tertiary aliphatic amines?
Account for the following.
pKb of aniline is more than that of methylamine.
Account for the following.
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Account for the following.
Ethylamine is soluble in water whereas aniline is not.
Arrange the following.
In increasing order of boiling point C6H5OH, (CH3)2NH, C2H5NH2.
How will you prepare propan-1-amine from butane nitrile?
How will you prepare propan-1-amine from 1-nitropropane?
How will you convert diethylamine into N, N-diethyl acetamide?
How will you convert diethylamine into N-nitrosodiethylamine?
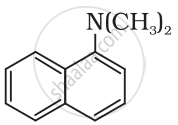
The following amine can be classified as:
Arrange the following in the increasing order of their basic strength:
C2H5NH2, C6H5NH2, (C2H5)2NH
Complete the following reaction.
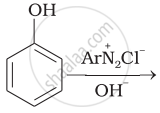
The amine formed from an amide by mean of bromine and alkali has how many number of carbon atoms?
Classify the following amine as primary, secondary or tertiary:

Among the following, which is the strongest base?
Write short note on the following:
Ammonolysis
Write a short note on the following.
Ammonolysis
Define Amines.
Write a short note on the following
Ammonolysis
Write a short note on the following.
Ammonolysis
Account for the following:
Aniline does not undergo Friedel-Crafts reaction.
Write short note on the following.
Ammonolysis
Write short note on the following:
Ammonolysis
Write a short note on the following.
Ammonolysis
Write a short note on the following.
Ammonolysis
