Advertisements
Advertisements
प्रश्न
Ketone : –CO– : : Ester : _______
उत्तर
Ketone : –CO– : : Ester : –COO–
APPEARS IN
संबंधित प्रश्न
Ethanoic acid has a .................... odour.
(a) Rotten eggs
(b) Pungent
(c) Vinegar-like
(d) Mild
Write three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate. Write balanced chemical equation in each case. Write the name of the reactants and the products other ethanoic acid and sodium ethanoate in each case.
What is the common name of ethanoic acid?
Fill in the following blank with a suitable word:
The organic acid present in vinegar is ______.
What happens when propanoic acid is warmed with methanol in the presence of a few drops of concentrated sulphuric acid? Write equation of the reaction involved.
What type to compound is CH3COOH?
Consider the following organic compound:
CH3OH, C2H5OH, CH3COCH3, CH3COOH, C2H5COOH, C4H9COOC2H5, CH4, C2H6, CH3CHO, HCHO
Out of these compound:
Which compound is added to alcohol to denature it?
Give two tests to show that CH3COOH is acidic in nature ?
Explain the following term with an example.
Oxidant
Fill in the blank with appropriate word/words.
Denatured alcohol is a mixture of _____ and _______
Give balanced chemical equations for the following conversion:
Calcium carbide to ethyne
CH3–CH2–CHO : propanal : : CH3–COOH : _______
Write the molecular formula of the given compound.
Sodium ethanoate
What are catalysts?
Observe the figure and write the answers to the following questions.

- Write the name of the reaction shown in the following figure.
- Write the above chemical reaction in the form of a balanced equation.
- Write the name of the product produced in the above reaction, write a use.
- Write the name of the catalyst used in the above reaction.
Anita added a drop each of diluted acetic acid and diluted hydrochloric acid on pH paper and compared the colors. Which of the following is the correct conclusion?
Raina while doing certain reactions observed that heating of substance ‘X’ with a vinegar-like smell with a substance ‘Y’ (which is used as an industrial solvent.) in the presence of conc. Sulphuric acid in a water bath gives a sweet-smelling liquid ‘Z’ having molecular formula C4H8O2. When heated with caustic soda (NaOH), ‘Z’ gives back the sodium salt and the compound ‘Y’.
Identify ‘X’, ‘Y’, and ‘Z’. Illustrate the changes with the help of suitable chemical equations.
Observe the diagram given below and answer the questions:
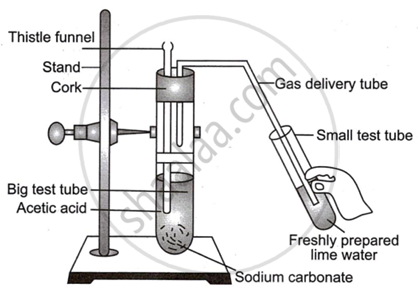
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
Give the balanced chemical equation of the reaction.
Oxidation of ethanol by acidified potassium dichromate.
