Advertisements
Advertisements
प्रश्न
On acid hydrolysis, propane nitrile gives.
पर्याय
Propanal
Acetic acid
Propionamide
Propanoic acid
उत्तर
Propanoic acid
APPEARS IN
संबंधित प्रश्न
Identify ‘A' and ‘B’ in the following reaction :
C6H5MgBr + C02 `(`> ‘A’ `(PCl_5)/()`> ‘B’
Predict the products of the following reactions:
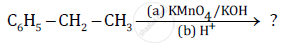
Name the reagents used in the following reactions:

Show how the following compound can be converted to benzoic acid.
Ethylbenzene
Show how the following compound can be converted to benzoic acid.
Acetophenone
Show how the following compound can be converted to benzoic acid.
Bromobenzene
Show how the following compound can be converted to benzoic acid.
Phenylethene (Styrene)
An organic compound (A) (molecular formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-ene. Write equations for the reactions involved.
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
m-Nitrobenzoic acid
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
p-Nitrobenzoic acid
How will you prepare the given compound from benzene? You may use any inorganic reagent and any organic reagent having not more than one carbon atom.
Phenylacetic acid
Complete the synthesis by giving missing starting material, reagent or product.
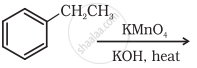
Name the reagents used in the following reactions:
The functional group present in triacylglycerol is _______.
Which is the most suitable reagent for the following conversion?
\[\begin{array}{cc}
\phantom{....................}\ce{O}\phantom{.....................................}\ce{O}\phantom{.}\\
\phantom{....................}||\phantom{......................................}||\phantom{.}\\
\phantom{}\ce{CH3 - CH = CH - CH2 - C - CH3 -> CH3 - CH = CH - CH2 - C - OH}\phantom{.}
\end{array}\]
Match the reactions given in Column I with the suitable reagents given in Column II.
| Column I (Reactions) |
Column II (Reagents) |
| (i) Benzophenone Diphenylmethane | (a) \[\ce{LiAlH4}\] |
| (ii) Benzaldehyde 1-Phenylethanol | (b) \[\ce{DIBAL-H}\] |
| (iii) Cyclohexanone Cyclohexanol | (c) \[\ce{Zn(Hg)/Conc. HCl}\] |
| (iv) Phenyl benzoate Benzaldehyde | (d) \[\ce{CH3MgBr}\] |
Assertion: Aldehydes and ketones, both react with Tollen’s reagent to form silver mirror.
Reason: Both, aldehydes and ketones contain a carbonyl group.
Substitution of one alkyl group by replacing hydrogen of primary amines
Match List - I with List - II.
| List - I | List - II | ||
| (a) |  \[\ce{->[CO,HCl][Anhyd. AlCl3/CuCl]}\] \[\ce{->[CO,HCl][Anhyd. AlCl3/CuCl]}\] |
(i) | Hell-Volhard-Zelinsky reaction |
| (b) |
\[\begin{array}{cc}
\ce{O}\phantom{.................}\\ ||\phantom{.................}\\ \ce{R - C - CH3 + NaOX ->} \end{array}\] |
(ii) | Gattermann-Koch reaction |
| (c) | \[\ce{R - CH2 - OH + R'COOH ->[Conc. H2SO4]}\] | (iii) | Haloform reaction |
| (d) | \[\ce{R - CH2COOH ->[(i) X2/Red P][(ii) H2O]}\] | (iv) | Esterification |
Choose the correct answer from the options given below.
The end product Y in the sequence of reaction:
\[\ce{RX ->[CN^-] X ->[NaOH] Y}\] is:
A compound 'X' with molecular formula C3H8O can be oxidised to a compound 'Y' with the molecular formula C3H6O2 'X' is most likely to be ______.
Fill in the blanks by choosing the appropriate words from those given in the brackets:
[stable, low, aldehyde, unstable, 6, 4, ethane, Clemmensen’s, 2, 3, carboxylic acid, high, propane, Rosenmund's]
The primary alcohols are easily oxidised first into ______ and then into ______.
