Advertisements
Advertisements
प्रश्न
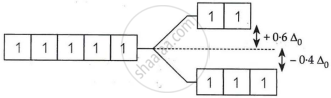
On the basis of crystal field theory, write the electronic configuration for the d5 ion with a weak ligand for which Δ0 < P.
उत्तर
For weak field ligand Δ0 < P i.e., pairing will not occur for d5 ion.
संबंधित प्रश्न
On the basis of crystal field theory, write the electronic configuration for d4 ion if Δ0 > P.
How are the following conversions carried out?
Benzoic acid into metanitrobenzoic acid.
Why are low spin tetrahedral complexes rarely observed?
The CFSE for octahedral \[\ce{[CoCl6]^{4-}}\] is 18,000 cm–1. The CFSE for tetrahedral \[\ce{[CoCl4]^{2-}}\] will be ______.
Atomic number of \[\ce{Mn, Fe, Co}\] and Ni are 25, 26, 27 and 28 respectively. Which of the following outer orbital octahedral complexes have same number of unpaired electrons?
(i) \[\ce{[MnCl6]^{3-}}\]
(ii) \[\ce{[FeF6]^{3-}}\]
(iii) \[\ce{[CoF6]^{3-}}\]
(iv) \[\ce{[Ni(NH3)6]^{2+}}\]
On the basis of crystal field theory explain why Co(III) forms paramagnetic octahedral complex with weak field ligands whereas it forms diamagnetic octahedral complex with strong field ligands.
\[\ce{CuSO4 . 5H2O}\] is blue in colour while \[\ce{CuSO4}\] is colourless. Why?
[Ni(H2O)6]2+ (aq) is green in colour whereas [Ni(H2O)4 (en)]2+ (aq)is blue in colour, give reason in support of your answer.
Considering crystal field theory, strong-field ligands such as CN–:
On the basis of Crystal Field theory, write the electronic configuration for the d5 ion with a strong field ligand for which Δ0 > P.
