Advertisements
Advertisements
प्रश्न
Silicon is a metallic element.
पर्याय
Right
Wrong
उत्तर
Silicon is a metallic element- Wrong
APPEARS IN
संबंधित प्रश्न
Choose the word or phrase from the brackets which correctly complete the following statement:
Metals are good ______ (oxidizing agents/reducing agents) because they are electron ______ (acceptors/donors).
Give the trend in metallic character:
(i) across the period left to right
Give the trend in metallic character:
down the group top to bottom.
Name the periodic property which relates to the character of element which loses one or more electrons when supplied with energy.
How do the following change on moving from left to right in a period of the periodic table?
Give examples in support of your answer.
Nature of oxides of the elements ?
iii) Observe the figure and answer the following questions.
a) Identify the block shown by box A and write an electronic configuration of any one element of this block.
b) Identify the block of element denoted by letter B and write its period number.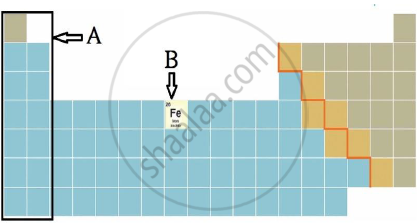
For the main group of the periodic table, the metallic properties of the elements vary approximately with their position as shown in the table.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| H | He | ||||||
| A | B | ||||||
| C | D |
Will the most metallic element be found at A,B,C or D ?
Select the correct answer
Configuration of an element is 2, 8, 1. Which of the following statement is true?
If an element is in group 7 is it likely to be metallic or non metallic in character?
Supply the missing word from the words given in brackets.
If an element has one electron in its outermost energy level, then it is likely to be _ (metallic, non metallic)
Identify the metallic oxide which is amphoteric in nature.
The lightest liquid metal is ______
Three elements B, Si and Ge are
Identify the elements with the following property and arrange them in increasing order of their reactivity
- An element which is a soft and reactive metal
- The metal which is an important constituent of limestone
- The metal which exists in liquid state at room temperature
Choose the odd one out and write the reason:
Arrange the following as per instruction given in the bracket.
Cs, Na, Li, K, Rb (increasing metallic character)
Metals are good ______ because they are electron ______.
Arrange:
Be, Li, C, B, N, O, F (in increasing metallic character).
A group of elements in the periodic table is given below (Boron is the first member of the group and Thallium is the last).
Boron, Aluminium, Gallium, Indium, Thallium
Answer the following question in relation to the above group of elements:
Will the elements in the group to the right of this boron group be more metallic or less metallic in character? Justify your answer.
