Advertisements
Advertisements
प्रश्न
When radiations of wavelength λ1 and λ2 are incident on certain photosensitive, such that E1 > E2 . Then Planck's constant 'h' is ......................... .
(C = Velocity of light).
पर्याय
`((E_1 - E_2)(lambda_1 - lambda_2))/(C(lambda_1 * lambda_2))`
`((E_1 - E_2)lambda_1C)/((lambda_1 - lambda_2)lambda_2)`
`((E_1 - E_2)lambda_1lambda_2)/(C(lambda_2 - lambda_1))`
`((lambda_1 - lambda_2)C)/((E_1 - E_2)lambda_1 * lambda_2)`
उत्तर
`((E_1 - E_2)lambda_1lambda_2)/(C(lambda_2 - lambda_1))`
APPEARS IN
संबंधित प्रश्न
The photoelectric threshold wavelength of a metal is 230 nm. Determine the maximum kinetic energy in joule and in eV of the ejects electron for the metal surface when it is exposed to a radiation of wavelength 180 nm.
[Planck’s constant : h = 6.63 * 10-34 Js, Velocity of light : C = 3 * 108 m/s.]
Find the value of energy of electron in eV in the third Bohr orbit of hydrogen atom.
(Rydberg's constant (R) = 1· 097 x 107m - 1,Planck's constant (h) =6·63x10-34 J-s,Velocity of light in air (c) = 3 x 108m/ s.)
Find the wave number of a photon having energy of 2.072 eV
Given : Charge on electron = 1.6 x 10-19 C,
Velocity of light in air = 3 x 108 m/s,
Planck’s constant = 6.63 x 10-34 J-s.
Write Einstein's photoelectric equation and mention which important features in photoelectric effect can be explained with the help of this equation.
The maximum kinetic energy of the photoelectrons gets doubled when the wavelength of light incident on the surface changes from λ1 to λ2. Derive the expressions for the threshold wavelength λ0 and work function for the metal surface.
A proton and a deuteron are accelerated through the same accelerating potential. Which one of the two has less momentum?
Give reasons to justify your answer.
Einstein's photoelectric equation is:
a) `E_"max" = hlambda - varphi_0`
b) `E_"max"= (hc)/lambda varphi_0`
c) `E_"max" = hv + varphi_0`
d) `E_"max" = (hv)/lambda + varphi_0`
With reference to the photoelectric effect, define threshold wavelength
Write the basic features of photon picture of electromagnetic radiation on which Einstein’s photoelectric equation is based.
Write Einstein’s photoelectric equation.
According to Einstein’s model, the threshold frequency for a metal having work function ϕ0 is given by _________.
Calculate the maximum kinetic energy of photoelectrons emitted by a metal (work function = 1.5 eV) when it is illuminated with light of wavelength 198 nm.
In an inelastic collision, which of the following does not remain conserved?
According to Einstein's photoelectric equation, the plot of the kinetic energy of the emitted photoelectrons from a metal Vs the frequency of the incident radiation gives as straight the whose slope:
A 200 W sodium street lamp emits yellow light of wavelength 0.6 µm. Assuming it to be 25% efficient in converting electrical energy to light, the number of photons of yellow light it emits per second is:
Who indirectly determined the mass of the electron by measuring the charge of the electrons?
What will be wavelength of a photon of momentum 6.6 × 10–24 kgms–1?
What is the momentum of photon of energy 3 mev in kg ms-1?
Which of the following is/are true for cathode ray
The wavelength of matter is independent of
- Calculate the energy and momentum of a photon in a monochromatic beam of wavelength 331.5 nm.
- How fast should a hydrogen atom travel in order to have the same momentum as that of the photon in part (a)?
- Calculate the frequency of a photon of energy 6.5 × 10−19 J.
- Can this photon cause the emission of an electron from the surface of Cs of work function 2.14 eV? If yes, what will be the maximum kinetic energy of the photoelectron?
How does stopping potential in photoelectric emission vary if the intensity of the incident radiation increases?
The graphs below show the variation of the stopping potential VS with the frequency (ν) of the incident radiations for two different photosensitive materials M1 and M2.
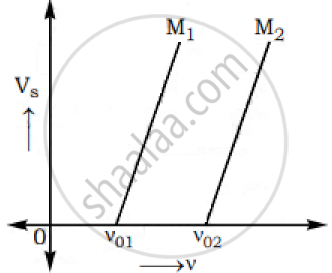
Express work function for M1 and M2 in terms of Planck’s constant(h) and Threshold frequency and charge of the electron (e).
If the values of stopping potential for M1 and M2 are V1 and V2 respectively then show that the slope of the lines equals to `(V_1-V_2)/(V_(01)-V_(02))` for a frequency,
ν > ν02 and also ν > ν01
