Advertisements
Advertisements
प्रश्न
Using valence bond theory, predict the hybridization and magnetic character of the following:
[CoF6]3– [Atomic number of Co = 27]
उत्तर
[CoF6]3– orbitals of Co2+ ion:
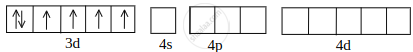
Co2+ undergoing sp3d2 hybridisation:
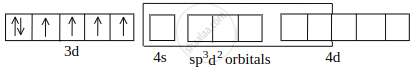
[CoF6]3– outer orbital or high-spin complex:
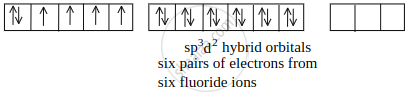
Hybridization = sp3d2
Magnetic Nature = Paramagnetic
संबंधित प्रश्न
Predict the number of unpaired electrons in the square planar [Pt(CN)4]2− ion.
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[Fe(CN)6]4−
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Write the hybridisation and number of unpaired electrons in the complex `[CoF_6]^(3-)`. (Atomic No. of Co = 27)
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[FeCl6]^{4-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
If orbital quantum number (l) has values 0, 1, 2 and 3, deduce the corresponding value of principal quantum number, n.
When the hybridization state of carbon changes from sp3 to sp2 and finally to sp, the angle between hybridized orbital will
As the s-character of hybridised orbital increases, the bond angle
Valence bond theory is based on the assumption that the bonds formed between the metal ions and ligands are ______
Write the hybridisation and magnetic behaviour of [CoF6]3−.
[Given: Atomic number of Co = 27]
