Advertisements
Advertisements
प्रश्न
Predict the number of unpaired electrons in the square planar [Pt(CN)4]2− ion.
उत्तर
78Pt is located in group 10 of the periodic table and its configuration is 5d96s1. Hence the configuration of Pt2+ is d8.
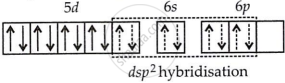
The hybridisation for square planar structure is dsp2. Since the unpaired electrons in 5d pair up to form a vacant d-orbital for dsp2 hybridisation, there are no unpaired electrons.
APPEARS IN
संबंधित प्रश्न
On the basis of valence bond theory explain the nature of bonding in [CoF6]3 ion.
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why? (Atomic number of Ni = 28)
Explain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2− is diamagnetic. Explain why?
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[Co(C2O4)3]3−
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[CoF6]3−
Explain the geometry of `[Co(NH_3)_6]^(3+)` on the basis of hybridisation. (Z of Co = 27)
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Cr(H2O)6]^{3+}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Write the hybridization and shape of the following complexes:
[Ni(CN)4]2−
In a coordination entity, the electronic configuration of the central metal ion is t2g3 eg1
Is the coordination compound a high spin or low spin complex?
Which of the following methods is used for measuring bond length?
Which of the following has square planar structures?
What is the no. of possible isomers for the octahedral complex [Co(NH3)2(C2O4)2]?
In Fe(CO)5, the Fe – C bond possesses
Using Valence bond theory, explain the following in relation to the paramagnetic complex [Mn(CN)6]3-
- type of hybridization
- magnetic moment value
- type of complex – inner, outer orbital complex
According to the valence bond theory, the hybridization of central metal atom is dsp2 for which one of the following compounds?
Write the hybridisation and magnetic behaviour of [CoF6]3−.
[Given: Atomic number of Co = 27]
