Advertisements
Advertisements
प्रश्न
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why? (Atomic number of Ni = 28)
Why is [NiCl4]2– paramagnetic but [Ni(CO)4] is diamagnetic? (At. nos. : Cr = 24, Co = 27, Ni = 28)
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why?
उत्तर १
In [Ni(CO)4], nickel is in zero oxidation state, whereas in [NiCl4]2− it is in +2 oxidation state. In the presence of CO ligand, the unpaired d-electrons of nickel get paired, but Cl– being a weak ligand is not able to pair the unpaired electrons. Hence, there is no unpaired electron present in [Ni(CO)4], so it is diamagnetic and due to the presence of unpaired electrons in [NiCl4]2−, it is paramagnetic.
उत्तर २
In [NiCl4]2−, the oxidation state of Ni is +2. Chloride is a weak field ligand and does not cause pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, two unpaired electrons are present in the valence d-orbitals of Ni, which impart paramagnetic character to the complex. On the other hand, carbonyl is a strong field ligand and causes pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, no unpaired electrons are present and hence, the complex is diamagnetic.
उत्तर ३
[NiCl4]2− and [Ni(CO)4] both are tetrahedral. But their magnetic characters are different. This is due to difference in the nature of ligands.
Ni+2 = [Ar] 4s03d8
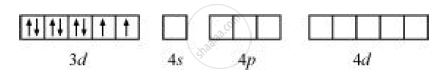
Ni+2 has 2 unpaired electrons hence, this complex is paramagnetic.
In Ni(CO)4, Ni is in the zero oxidation state i.e., it has a configuration of 3d84s2.
Ni = [Ar] 4s2 3d8
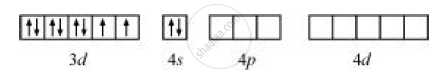
But CO is a strong field ligand. Therefore, it causes the pairing of unpaired 3d electrons. Also, it causes the 4s electrons to shift to the 3d orbital, thereby giving rise to sp3 hybridization. Since no unpaired electrons are present in this case, [Ni(CO)4] is diamagnetic.
Notes
Students should refer to the answer according to their questions.
APPEARS IN
संबंधित प्रश्न
Predict the number of unpaired electrons in the square planar [Pt(CN)4]2− ion.
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[Fe(CN)6]4−
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[FeF6]3−
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Mn(CN)6]^{3-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
If orbital quantum number (l) has values 0, 1, 2 and 3, deduce the corresponding value of principal quantum number, n.
Which of the following methods is used for measuring bond length?
Which of the following has square planar structures?
In Fe(CO)5, the Fe – C bond possesses
Using valence bond theory, predict the hybridization and magnetic character of the following:
[CoF6]3– [Atomic number of Co = 27]
The magnetic moment of [NiCl4]2− is ______.
[Atomic number: Ni = 28]
