Commerce (English Medium)
Science (English Medium)
Arts (English Medium)
Academic Year: 2013-2014
Date: मार्च 2014
Advertisements
Why is adsorption always exothermic?
Chapter: [0.05] Surface Chemistry
Name the method used for the refining of Nickel metal.
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Why does NO2 dimerise?
Chapter: [0.07] P - Block Elements
Based on molecular forces, what type of polymer is neoprene?
Chapter: [0.15] Polymers
What are the products of hydrolysis of maltose?
Chapter: [0.1] Biomolecules
Write the structure of 4-chloropentan-2-one.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Identify the chiral molecule in the following pair :

Chapter: [0.06] Haloalkanes and Haloarenes
The conversion of primary aromatic amines into diazonium salts is known as ___________
Chapter: [0.09] Amines
Write the names of monomers used for getting the following polymers
Terylene
Chapter:
Write the names and structure of the monomers of the following polymers: Nylon-6, 6
Chapter: [0.15] Polymers
Describe the roles of the following: SiO2 in the extraction of copper from copper matte
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Describe the roles of the following: NaCN in froth floatation process
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Complete the following equations: Ag+PCl5 →
Chapter: [0.07] P - Block Elements
Complete the following equations: CaF2+H2SO4 →
Chapter: [0.07] P - Block Elements
Draw the structures of the following molecules: XeF4
Chapter: [0.07] P - Block Elements
Draw the structures of the following:
(1) HClO4
(2) H3PO3
Chapter: [0.07] P - Block Elements
Advertisements
Write the type of magnetism observed when the magnetic moments are oppositely aligned and cancel out each other.
Chapter: [0.01] Solid State
Which stoichiometric defect does not change the density of the crystal?
Chapter: [0.01] Solid State
Define terms: Fuel cell
Chapter: [0.02] Electrochemistry
Define limiting molar conductivity.
Chapter: [0.02] Electrochemistry
Write the mechanism of the following reaction :
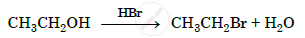
Chapter: [0.07] Alcohols, Phenols and Ethers
For a chemical reaction R → P, the variation in the concentration (R) vs. time (t) plot is given as:
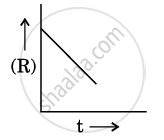
(i) Predict the order of the reaction.
(ii) What is the slope of the curve ?
(iii) Write the unit of rate constant for this reaction.
Chapter: [0.03] Chemical Kinetics
An element with density 2.8 g cm–3 forms a f.c.c. unit cell with edge length 4 x 10–8 cm. Calculate the molar mass of the element.
(Given : NA = 6.022 x 1023 mol –1)
Chapter: [0.01] Solid State
Write the equation involved in the following reaction:
Reimer-Tiemann reaction
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the equations involved in the following reactions : Williamson synthesis
Chapter: [0.07] Alcohols, Phenols and Ethers
Define the following term:
Glycosidic linkage
Chapter: [0.1] Biomolecules
Define the following terms:
Invert sugar
Chapter: [0.1] Biomolecules
Define the following terms:
Oligosaccharides
Chapter: [0.1] Biomolecules
On the occasion of World Health Day, Dr. Satpal organized a ‘health camp’ for the poor farmers living in a nearby village. After check-up, he was shocked to see that most of the farmers suffered from cancer due to regular exposure to pesticides and many were diabetic. They distributed free medicines to them. Dr. Satpal immediately reported the matter to the
National Human Rights Commission (NHRC). On the suggestions of NHRC, the government decided to provide medical care, financial assistance, setting up of super-speciality hospitals for treatment and prevention of the deadly disease in the affected villages all over India.
(i) Write the values shown by
(a) Dr. Satpal
(b) NHRC
(ii) What type of analgesics are chiefly used for the relief of pains of terminal cancer ?
(iii) Give an example of artificial sweetener that could have been recommended to diabetic patients
Chapter: [0.16] Chemistry in Everyday Life
Give reason for the following:
Primary amines have higher boiling point than tertiary amines.
Chapter: [0.09] Amines
Give reasons for the following:
Aniline does not undergo Friedel- Crafts reaction.
Chapter: [0.09] Amines
Give reasons for the following: (CH3)2NH is more basic than (CH3)3N in an aqueous solution.
Chapter: [0.09] Amines
Give the structures of A, B and C in the following reactions :
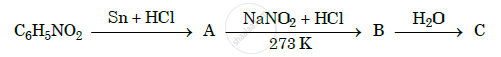
Chapter: [0.09] Amines
Give the structures of A, B and C in the following reactions :
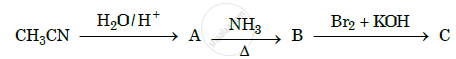
Chapter: [0.09] Amines
Draw the structures of major monohalo products in each of the following reactions :
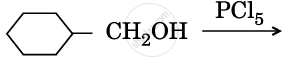
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Draw the structures of major monohalo products in each of the following reactions :
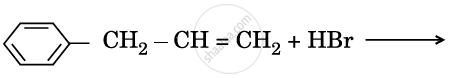
Chapter:
Which halogen compound in each of the following pairs will react faster in SN2 reaction CH3Br or CH3I
Chapter: [0.07] P - Block Elements
Which halogen compound in each of the following pairs will react faster in SN2 reaction
(CH3)3 C – Cl or CH3 – Cl
Chapter: [0.07] P - Block Elements
Advertisements
Calculate ΔrG° for the reaction
Mg (s) + Cu2+ (aq) → Mg2+ (aq) + Cu (s)
Given : E°cell = + 2.71 V, 1 F = 96500 C mol–1
Chapter: [0.02] Electrochemistry
The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume :
SO2Cl2 (g) → SO2 (g) + Cl2 (g)
| Experiment | Time/s–1 | Total pressure/atm |
| 1 | 0 | 0.4 |
| 2 | 100 | 0.7 |
Calculate the rate constant.
(Given : log 4 = 0.6021, log 2 = 0.3010)
Chapter: [0.03] Chemical Kinetics
What are their different types emulsions? Give one example of each type.
Chapter: [0.05] Surface Chemistry
Give reasons for the following : (CH3)3 P = O exists but (CH3)3 N = O does not.
Chapter: [0.07] P - Block Elements
Give reasons for the following : Oxygen has less electron gain enthalpy with negative sign than sulphur.
Chapter: [0.07] P - Block Elements
Give reasons for the following : H3PO2 is a stronger reducing agent than H3PO3.
Chapter: [0.07] P - Block Elements
Write the IUPAC name of the complex [Cr(NH3)4 Cl2]Cl.
Chapter: [0.05] Coordination Compounds
What type of isomerism is exhibited by the complex [Co(en)3]3+?
(en = ethane-1,2-diamine)
Chapter: [0.05] Coordination Compounds
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why? (Atomic number of Ni = 28)
Chapter: [0.05] Coordination Compounds
Write the products formed when CH3CHO reacts with the following reagents : HCN
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the products formed when CH3CHO reacts with the following reagents : H2N – OH
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the products formed when CH3CHO reacts with the following reagents: CH3CHO in the presence of dilute NaOH
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol
Chapter: [0.07] Alcohols, Phenols and Ethers
Propanal and Propanone
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Account for the following : Cl – CH2COOH is a stronger acid than CH3COOH.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Why carboxylic acid does not give reactions of carbonyl group?
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the chemical equations to illustrate the following name reactions : Rosenmund reduction
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the chemical equations to illustrate the following name reaction:
Cannizzaro’s reaction
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Out of CH3CH2 – CO – CH2 – CH3 and CH3CH2 – CH2 – CO – CH3, which gives iodoform test?
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Define the following terms : Molarity
Chapter: [0.01] Solutions
Define the following terms : Molal elevation constant (Kb)
Chapter: [0.01] Solutions
A solution containing 15 g urea (molar mass = 60 g mol–1) per litre of solution in water has the same osmotic pressure (isotonic) as a solution of glucose (molar mass = 180 g mol–1) in water. Calculate the mass of glucose present in one litre of its solution.
Chapter: [0.01] Solutions
What type of deviation is shown by a mixture of ethanol and acetone? Give reason.
Chapter: [0.01] Solutions
A solution of glucose (molar mass = 180 g mol–1) in water is labelled as 10% (by mass). What would be the molality and molarity of the solution ? (Density of solution = 1.2 g mL–1)
Chapter: [0.01] Solutions
Complete the following equations:Cr2O72- + 2OH- →
Chapter: [0.04] d-block and f-block Elements
Complete the following equations : MnO4- + 4H+ + 3e- →
Chapter: [0.04] d-block and f-block Elements
Account for the following :
Zn is not considered as a transition element.
Chapter: [0.04] d-block and f-block Elements
How would you account for the following: Transition metals form complex compounds.
Chapter: [0.04] d-block and f-block Elements
Account for the following:
E° value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+.
Chapter: [0.04] d-block and f-block Elements
With reference to structural variability and chemical reactivity, write the differences between lanthanoids and actinoids
Chapter: [0.04] d-block and f-block Elements
Name a member of the lanthanoid series which is well known to exhibit +4 oxidation state.
Chapter: [0.04] d-block and f-block Elements
Complete the following equation : MnO4- + 8H+ + 5e- →
Chapter: [0.04] d-block and f-block Elements
Out of Mn3+ and Cr3+, which is more paramagnetic and why ?
(Atomic nos. : Mn = 25, Cr = 24)
Chapter: [0.04] d-block and f-block Elements
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2013 - 2014
Previous year Question paper for CBSE Class 12 -2014 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
