Advertisements
Advertisements
प्रश्न
What are hydrocarbons? Write the general formula of (i) saturated hydrocarbons, and (ii) unsaturated hydrocarbons and draw the structure of one hydrocarbon of each type. How can an unsaturated hydrocarbon be made saturated?
उत्तर
The compounds entirely consisting of carbons and hydrogens are known as Hydrocarbons. There are different categories in which hydrocarbons are divided out of which the two are:
(i) Saturated Hydrocarbons: The compounds of carbon having only single bonds between the carbon atoms are called saturated compounds. This includes alkanes, having a general formula CnH2n+2.
(ii) Unsaturated Hydrocarbons: The compounds of carbon having double and triple bonds between the carbon atoms are called unsaturated compounds. This includes alkenes and alkynes having general formula CnH2n and CnH2n-2, respectively.
Example
(i) Saturated hydrocarbon: C2H6 (Ethane)
Structure:
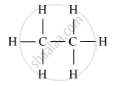
(i) Saturated Hydrocarbons: The compounds of carbon having only single bonds between the carbon atoms are called saturated compounds. This includes alkanes, having a general formula CnH2n+2.
(ii) Unsaturated Hydrocarbons: The compounds of carbon having double and triple bonds between the carbon atoms are called unsaturated compounds. This includes alkenes and alkynes having general formula CnH2n and CnH2n-2, respectively.
Example
(i) Saturated hydrocarbon: C2H6 (Ethane)
Structure:

(ii) (a) Unsaturated hydrocarbon: C2H4 (Ethene)
Structure:
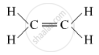
Structure:

(b) Unsaturated hydrocarbon: C2H2 (Ethyne)
Structure:
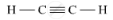
Structure:
shaalaa.com
या प्रश्नात किंवा उत्तरात काही त्रुटी आहे का?
संबंधित प्रश्न
Write the number of covalent bonds in the molecule of ethane.
Draw the electron dot structures for H2S.
Give a test that can be used to differentiate chemically between butter and cooking oil.
Write the electron-dot structures for ethyne.
Name a chemical reaction which is characteristic of unsaturated hydrocarbons (like alkenes and alkynes).
Which of the two is better for our health : butter or vegetable oil? Why?
The name of the compound CH3 — CH2 — CHO is
Identify and name the functional groups present in the following compounds.
- \[\begin{array}{cc}
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C - C - OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{....}\\
\phantom{...}|\phantom{....}|\phantom{....}||\phantom{....}\\
\ce{H - C - C - C - OH}\\
\phantom{...}|\phantom{....}|\phantom{.........}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{....}\ce{H}\phantom{....}\ce{O}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\phantom{....}|\phantom{....}|\phantom{....}||\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{H}\\
\phantom{....}|\phantom{.....}|\phantom{........}|\phantom{....}|\phantom{....}\\
\phantom{....}\ce{H}\phantom{....}\ce{H}\phantom{........}\ce{H}\phantom{..}\ce{H}\phantom{.....}\\
\end{array}\] - \[\begin{array}{cc}
\phantom{......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{......}\\
\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}\\
\ce{H - C - C - C = C - H}\\
\phantom{....}|\phantom{....}|\phantom{.............}\\
\phantom{....}\ce{H}\phantom{...}\ce{H}\phantom{.............}
\end{array}\]
What is the role of metal or reagents written on arrows in the given chemical reactions?
- \[\begin{array}{cc}
\ce{CH3}\phantom{.....}\ce{CH3}\phantom{.........}\ce{CH3}\phantom{..}\ce{CH3}\phantom{..........}\\
\phantom{...}\backslash\phantom{......}/\phantom{................}|\phantom{.....}|\phantom{............}\\
\ce{C} = \ce{C} + \ce{H2} \overset{\ce{Ni}}{\rightarrow} \ce{CH3 - C - C - CH3}\\
\phantom{.}/\phantom{........}\backslash\phantom{..............}|\phantom{....}|\phantom{.........}\\
\ce{CH3}\phantom{....}\ce{CH3}\phantom{............}\ce{H}\phantom{...}\ce{H}\phantom{........}
\end{array}\] - \[\ce{CH3COOH + CH3CH2OH \overset{\ce{Conc. H2SO4}}{\rightarrow}CH3COOC2H5 + H2O}\]
- \[\ce{CH3CH2OH}\ce{->[Alk.KMnO4][Heat]CH3COOH}\]
Draw the electron dot structure for ethyne.
