Advertisements
Advertisements
प्रश्न
What happens when
Caustic soda solution is added to Cu(NO3)2 solution and product boiled.
उत्तर
When Caustic soda solution is added to Cu(NO3)2 solution, the following reaction takes place:
Cu(NO3)2(aq) + 2NaOH(aq) → Cu(OH)2(S) + 2NaNO3(aq)
When we heat it further, the greenish blue copper hydroxide decomposes to form slightly black ppt. of copper oxide.
\[\ce{Cu(OH)2(S) ->[\Delta] CuO(s) + H2O(g)}\]
APPEARS IN
संबंधित प्रश्न
Name the gas that is produced in the given cases :
Sulphur is oxidized by concentrated nitric acid.
Conc. Nitric acid prepared in laboratory is yellow in colour why? How is this colour removed?
Identify the substance underlined:
The dilute acid which is an oxidizing agent.
What is the oxidizing agent in ostwald process ?
Write the balanced equation for the following:
Action of concentrated nitric acid on copper.
Write the equation to show the reaction between the following:
Between copper and concentrated nitric acid.
Write the equation of the following reaction.
Sulphur can be converted to sulphuric acid using concentrated nitric acid.
Write equation to show the reaction between the following:
Copper and concentrated nitric acid.
Fill in the blank using the appropriate words given below:
Hot, concentrated nitric add reacts with sulphur to form ______.
The diagram given below is a representation of the Industrial preparation of Nitric acid by Ostwald’s process. With respect to the process answer the following questions:
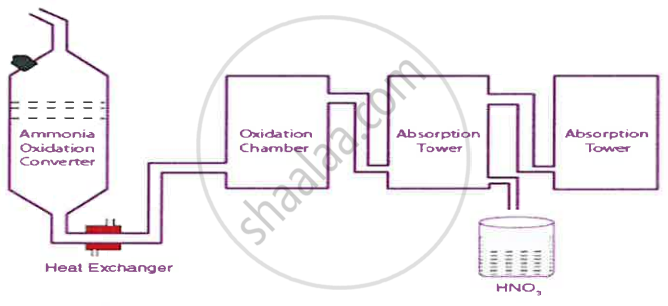
- Write the temperature and the catalyst required during the catalytic oxidation of ammonia.
- Give balanced chemical equation for the reaction occurring duringthe conversion of nitrogen dioxide to nitric acid.
