Advertisements
Advertisements
प्रश्न
Why covalent compounds are different from ionic compounds?
उत्तर
Ionic compounds, on the other hand, are the compounds that are formed by the complete transfer of valence electrons between the constituent atoms. For example, a molecule of sodium chloride (NaCl) is formed when the sodium atom donates its one valence electron to the chlorine atom.
संबंधित प्रश्न
Choose the correct answer from the options given below:
Which of the following is a common characteristic of a covalent compound?
1) high melting point
2) consists of molecules
3) always soluble in water
4) Conducts electricity when it is in the molten state
Write any three features and give two examples of covalent compounds
Name the hardest natural substance known.
What type of chemical bond is formed between carbon and bromine?
Compare the properties of ionic compounds and covalent compounds.
The organic compound prepared by Wohler from an inorganic compound called ammonium cyanate was:
(a) glucose
(b) urea
(c) uric acid
(d) vinegar
The following structural formula belongs to which carbon compound?
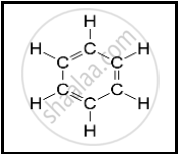
Choose the correct answer from the options given below
Condition favorable for formation of a covalent bond is
Fill in the blank with correct word from the bracket.
Melting and boiling points of covalent compounds are generally ______ (low, high).
Explain the bonding in methane molecule using electron dot structure.
Draw an electron dot diagram to show the formation of the following compound.
Magnesium chloride [ H=1, C=6, Mg=12, Cl=17].
Explain the following:
Water is a polar covalent molecule.
Complete the following activity.
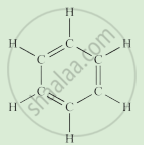
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
 |
List two differences between the properties exhibited by covalent compounds and ionic compounds.
Give an example for each of the following statement
A compound in which two Covalent bonds are formed.
When ethyl alcohol and acetic acid are mixed, the resulting ester has a chemical formula ______.
Although metals form basic oxides, which of the following metals form an amphoteric oxide?
______ is an example of a covalent compound having a high melting point.
Molecular reactions are ______ in the covalent compound.
