Advertisements
Advertisements
प्रश्न
Why is it safer to use soap from the environmental point of view?
उत्तर
Soaps are biodegradable. The detergents are quite stable and are non-biodegradable because of branching in hydrocarbon chain hence cause water pollution. Therefore, it is safer to use soap from the environmental point of view.
APPEARS IN
संबंधित प्रश्न
Explain cationic detergents.
What is a soap ?
Write the chemical equation for preparing sodium soap from glyceryl oleate . Structural formulae of the compounds are given below.
(C17H32COO)3C3H5 – Glyceryl oleate
Following type of nom-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Identify the functional group (s) present in the molecule.
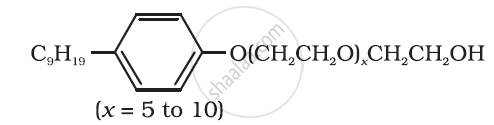
How soap is prepared?
Glycerol is added to soap. It functions ______.
What is the side product of soap industry? Give reactions showing soap formation.
Match the soaps given in Column I with items given in Column II.
| Column I | Column II |
| (i) Soap chips | (a) dried miniature soap bubbles |
| (ii) Soap granules | (b) small broken pieces of soap formed from melted soaps |
| (iii) Soap powder | (c) soap powder + abrasives + builders \[\ce{(Na2CO3,Na3PO4)}\] |
| (iv) Scouring soap | (d) soap powder + builders like \[\ce{Na2CO3}\] and \[\ce{Na3PO4}\] |
Match the detergents given in Column I with their uses given in Column II.
| Column I | Column II |
(i) 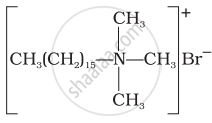 |
(a) Dishwashing powder |
(ii) 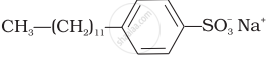 |
(b) Laundry soap |
| (iii) \[\ce{C17H33CO\overset{-}{O}\overset{+}{N}a + Na2CO3 + Rosin}\] | (c) Hair conditioners |
| (iv) \[\ce{CH3(CH2)16COO(CH2CH2O)nCH2CH2OH}\] | (d) Toothpaste |
Assertion: Sodium chloride is added to precipitate soap after saponification.
Reason: Hydrolysis of esters of long-chain fatty acids by alkali produces soap in colloidal form.
