Advertisements
Advertisements
प्रश्न
Why water get cooled in a ‘Surahi’ in hot season?
उत्तर
‘Surahi’ is made of clay which is porous (being not glazed) and so water oozes out (creeps out) and wets its entire surface. Form its outer surface, water get evaporated taking latent heat needed for evaporation from inside water as well as surrounding air and so the temperature of inside water falls.
APPEARS IN
संबंधित प्रश्न
What do you understand by the term latent heat?
- Which requires more heat: 1 g ice at 0℃ or 1 g water at 0℃ to raise its temperature to 10℃?
- Explain your answer in part (a).
Explain the following:
The surrounding become pleasantly warm when water in a lake starts freezing in cold countries.
When a liquid is getting converted into solid, the latent heat is ………………………………
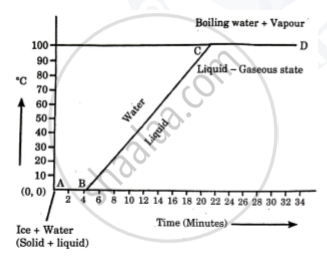
Explain the following temperature vs time graph.
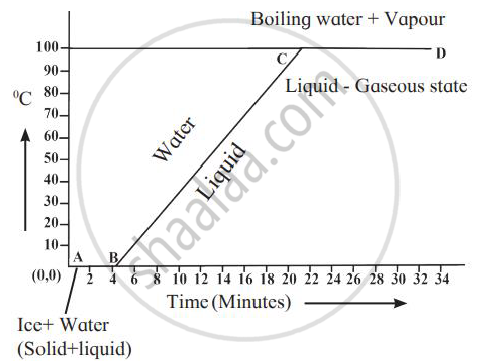
Explain the following temperature Vs. time graph:
A substance changes from its solid state to the liquid state when heat is supplied to it. What name is given to heat absorbed by the substance.
Explain why water is used in hot water bottles for fomentation and also as a universal coolant.
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
When 1 g of ice at 0 °C melts to form 1 g of water at 0 °C then, is the latent heat absorbed by the ice or given out by it?
Why do we feel much comfortable when we sit under a moving fan especially when our body is sweating?
What observation you will record and how will you determine the specific latent heat of fusion of ice?
If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.
(i) Find:
(a) The heat lost by steam in terms of m1
(b) The heat gained by ice in terms of m2
(ii) Form a heat equation find the ratio of m2 : m1
Specific latent heat of vaporization of steam = 2268 kJ/kg
Specific latent heat of fusion of ice = 336 kJ/kg
Specific heat capacity of water = 4200 J/kg°C
1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporization = 2260 J/g, calculate:
(i) the time taken for the temperature of water to rise from 25°C to its boiling point,
(ii) the mass of water which evaporates per minute from the boiling water.
Specific latent heat of vaporisation : J/kg : : specific heat : _______
Who introduced the term latent heat?
