Advertisements
Advertisements
प्रश्न
When a liquid is getting converted into solid, the latent heat is ………………………………
उत्तर
When a liquid is getting converted into solid, the latent heat is Released.
APPEARS IN
संबंधित प्रश्न
State two characteristics of a good thermion emitter.
State two factors upon which the rate of emission of thermions depends.
Write the approximate value of specific latent heat of ice.
Which has more heat: 1 g ice at 0℃ or 1g water 0℃? Give reason.
During transformation of liquid phase to solid phase, the latent heat is ______.
Explain the following temperature vs time graph.

What is meant by latent heat? How will the state of matter transform if latent heat is given off?
Explain the following temperature Vs. time graph:
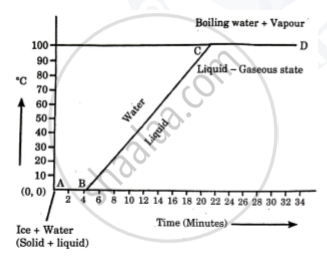
Define specific latent heat of vaporization of a substance.
Explain why water is used in hot water bottles for fomentation and also as a universal coolant.
Define the term ‘specific latent heat of fusion’ of a substance.
Explain the statement; “The specific latent heat of vaporization of wafer is 2260 × 103 J/kg”.
Derive an expression for the amount of heat given out or taken up, when its temperature falls or rises by t°C.
If pressure increases, the melting point of a substance ______.
Find the odd one out and give its explanation.
Write the name.
Products obtained when sugar is heated.
Write scientific reason.
Even if boiling water is constantly heated, its temperature does not rise.
For the same mass of ice and ice-cold water, why does ice produce more cooling than ice-cold water?
Calculate the total amount of heat energy required to melt 200 g of ice at 0°C to water at 100°C. (Specific latent heat of ice = 336 Jg-1, specific heat capacity of water = 4.2 Jg-1 °C-1)
