Advertisements
Advertisements
Question
When a liquid is getting converted into solid, the latent heat is ………………………………
Solution
When a liquid is getting converted into solid, the latent heat is Released.
APPEARS IN
RELATED QUESTIONS
State two factors upon which the rate of emission of thermions depends.
Explain the following:
The surrounding become pleasantly warm when water in a lake starts freezing in cold countries.
What is meant by latent heat? How will the state of matter transform if latent heat is given off?
Water expands on reducing its temperature below ______°C.
Give one consequence of the high specific latent heat of fusion of ice.
Why water get cooled in a ‘Surahi’ in hot season?
Define the term ‘specific latent heat of fusion’ of a substance.
Calculate the total amount of heat required to convert 100g ice at 0°C to steam at 100°C.
(Specific latent heat of fusion of ice = 336 J/g, specific latent heat of vaporization of steam = 2260 J/g, specific heat capacity of water = 4.2 J/g°C).
If pressure increases, the melting point of a substance ______.
When ice is converted into water : constant temperature : : before the water evaporates : _______
Specific latent heat of vaporisation : J/kg : : specific heat : _______
Find the odd one out and give its explanation.
Define boiling point of a liquid.
Write the name.
The phase in which solid substances are converted into liquid.
1 kg of dry air at a temperature of 40 °C can hold a maximum of 49 g of water vapour.
600 g of copper at 50°C is mixed with lOOOg water at 20°C. Find the final temperature of the mixture. The specific heat capacity of copper is 0.4 Jg-1°C-1 and that of water is 4.2 Jg-1°C-1
Specific latent heat of a substance ______.
The diagram below shows a cooling curve for a substance:
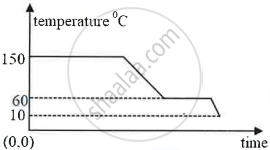
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
