Advertisements
Advertisements
प्रश्न
What is meant by latent heat? How will the state of matter transform if latent heat is given off?
उत्तर
- The heat energy required to change substance from one state to another at constant temperature is called as latent heat of a substance.
- When latent heat is given off from a substance, it causes the strengthening of the bonds between atoms or molecules causing a change of state of the substance.
- When a liquid substance gives off latent heat of fusion, strengthening of bonds results into pulling of atoms or molecules of liquid closer and eventually it changes into solid-state.
- Similarly, when a gaseous substance gives off latent heat of vaporisation, strengthening of bonds results into the pulling of atoms or molecules of gas closer and eventually it changes into a liquid state.
संबंधित प्रश्न
State any two measures to minimize the impact of global warming.
What is the energy absorbed during the phase change called?
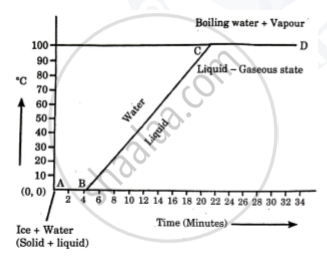
Explain the following temperature vs time graph.

Water expands on reducing its temperature below ______°C.
Explain the following temperature Vs. time graph:
Explain why water is used in hot water bottles for fomentation and also as a universal coolant.
Explain the meaning of the term latent heat. State its S. I. unit.
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
What do you understand by the ‘latent heat of vaporization’ of a substance?
Derive an expression for the amount of heat given out or taken up, when its temperature falls or rises by t°C.
If there is no Heat loss to the surroundings, the heat released by the condensation of m1 g of steam at 100°C into water at 100°C can be used to convert m2 g of ice at 0°C into water at 0°C.
(i) Find:
(a) The heat lost by steam in terms of m1
(b) The heat gained by ice in terms of m2
(ii) Form a heat equation find the ratio of m2 : m1
Specific latent heat of vaporization of steam = 2268 kJ/kg
Specific latent heat of fusion of ice = 336 kJ/kg
Specific heat capacity of water = 4200 J/kg°C
Write the name.
Products obtained when sugar is heated.
Write scientific reason.
The bottom of some steel utensils used for cooking is copper.
600 g of copper at 50°C is mixed with lOOOg water at 20°C. Find the final temperature of the mixture. The specific heat capacity of copper is 0.4 Jg-1°C-1 and that of water is 4.2 Jg-1°C-1
Observe the following graph and answer the following questions:

- What does the graph represent?
- What does the line AB represent?
- What does the line BC represent?
The amount of heat energy required to melt a given mass of a substance at its melting point without any rise in its temperature is called as the ______.
The diagram below shows a cooling curve for a substance:
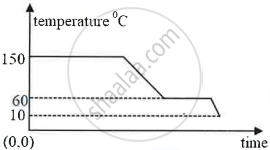
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
