Advertisements
Advertisements
प्रश्न
Write down what you understood from the following chemical reaction.
AgNO3(aq) + NaCI(aq) → AgCI ↓ + NaNO3(aq)
उत्तर
i. AgNO3 and NaCl are reactants and AgCl and NaNO3 are products.
ii. Reactants and the product NaNO3 are in aqueous form.
iii. Product AgCl is formed in the form of precipitate.
APPEARS IN
संबंधित प्रश्न
What is a balanced chemical equation? Why should a chemical equation be balanced?
Write the balanced chemical equation for the following reaction.
\[\ce{Aluminium + Copper chloride -> Aluminium chloride + Copper}\]
Give one example of a chemical reaction.
Correct and balance the following equation:
N + H  NH3
NH3
Balance the given equation:
H2O2  H2O + O2
H2O + O2
Balance the given equation:
AI(OH)3  AI2O3 +H2O
AI2O3 +H2O
Giving examples, state the difference between balanced and unbalanced chemical equations.
Aluminium burns in chlorine to form aluminium chloride (AlCl3). Write a balanced chemical equation for this reaction.
Write any two observations in an activity which may suggest that a chemical reaction has taken place. Give an example in support of your answer.
Potassium chlorate (KClO3) on heating forms potassium chloride and oxygen. Write a balanced equation for this reaction and indicate the evolution of gas.
Write balanced chemical equation with state symbols for the following reaction:
Sodium hydroxide solution reacts with hydrochloric acid solution to produce sodium chloride solution and water.
State one characteristic of the chemical reaction which takes place when quicklime is treated with water
State one characteristic of the chemical reaction which takes place when wax is burned in the form of a candle?
Is Burning of natural gas an endothermic reaction or an exothermic reaction?
Balance the following chemical equation:
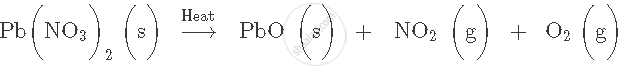
Write word equation for the following skeletal equation:
\[\ce{Zn + HCl -> ZnCl2 + H2}\]
Write word equation for the following skeletal equation:
\[\ce{CO + O2 -> CO2}\]
Write your observation for the following chemical reaction and name the product formed :
When an aqueous solution of sodium chloride is mixed with an aqueous solution of silver nitrate.
Write symbolic representation for the following word equation and balance them :
Carbon + Oxygen → Carbon dioxide.
Write symbolic representation for the following word equation and balance them :
Aluminium + Chlorine → Aluminium chloride.
Write symbolic representation for the following word equation and balance them :
Iron + Sulphur → Iron sulphide.
Balance the following equation. Also name the product formed.
`"H"_2 +"CI"_2 → "HCI"`
In the experiment for determining the percentage of water absorbed by raisins, we do the final weighing of the raisins after keeping them dipped in water for about one hour. For the accuracy of the result, the extra water from the surface of the soaked raisins is removed by
The step(s) necessary for determining the percentage of water absorbed by raisins is/are:
Balance the following equation:
MnO2 + HCl → MnCl2 + H2O + Cl2
Balance the following equation:
H2O + Cl2 → HCl + O2
Answer the following question:
1 g of copper powder was taken in a China dish and heated. What change takes place on heating? When hydrogen gas is passed over this heated substance, a visible change is seen in it. Give the chemical equations of reactions, the name and the color of the products formed in each case.
Write word equation for the following molecular equation:
\[\ce{CaCO3 + 2HCl ->[\triangle] CaCl2 + H2O + CO2 [g]}\]
Word equation:
State which characteristic does [g] in the above reaction indicate. The word equation of:
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – copper carbonate.
Name the following:
Two gases which react under pressure in presence of a catalyst at elevated temperatures to give a gaseous product.
Name the following:
A catalyst which increases the rate of a chemical reaction.
Balance the following simple equation:
K + CO2 → K2O + C
Balance the following simple equation:
H2 + O2 → H2O
Balance the following simple equation:
\[\ce{N2 + H2 <=> NH3}\]
Balance the following simple equation:
KBr + Cl2 → KCl + Br2
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
CaC2 + N2 → 2CaCN2 + C
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
NaOH + CO2 → Na2CO3 + H2O
Three beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statement(s) is(are) correct?
- In beakers A and B, exothermic process has occurred.
- In beakers A and B, endothermic process has occurred.
- In beaker C exothermic process has occurred.
- In beaker C endothermic process has occurred.
What are the steps involved in writing the skeletal equation?
