Advertisements
Advertisements
Question
Write down what you understood from the following chemical reaction.
AgNO3(aq) + NaCI(aq) → AgCI ↓ + NaNO3(aq)
Solution
i. AgNO3 and NaCl are reactants and AgCl and NaNO3 are products.
ii. Reactants and the product NaNO3 are in aqueous form.
iii. Product AgCl is formed in the form of precipitate.
APPEARS IN
RELATED QUESTIONS
State whether the following statement is true or false:
A chemical equation can be balanced easily by altering the formula of a reactant or product.
Balance the given equation:
BaCI2 + H2SO4  BaSO4 + HCI
BaSO4 + HCI
Write balanced chemical equation from the following information:
An aqueous calcium hydroxide solution (lime water) reacts with carbon dioxide gas to produce a solid calcium carbonate precipitate and water.
Aluminium burns in chlorine to form aluminium chloride (AlCl3). Write a balanced chemical equation for this reaction.
Substitute formulae for names and balance the following equation:
Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water and carbon dioxide gas.
With the help of an appropriate example, justify that some of the chemical reactions are determined by Evolution of a gas.
Give chemical equation for the reaction involved in the above case.
What do you understand by endothermic reactions?
Is Electrolysis of water an endothermic reaction or an exothermic reaction?
Balance the following chemical equation:
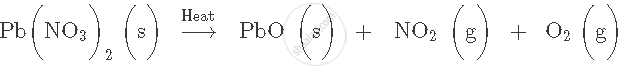
Express the following facts in the form of a balanced chemical equation:
"When a strip of copper metal is placed in a solution of silver nitrate, metallic silver is precipitated and a solution containing copper nitrate is formed".
Explain the following reaction with one suitable example .
Decomposition reaction
Name the type of chemical reaction shown by the following equation:
2Mg+ O2 → 2MgO
Name the type of chemical reaction shown by the following equation:
Fe + CuSO4 → FeSO4 +Cu
Why do we need to balance chemical equations?
Write word equation for the following skeletal equation:
\[\ce{FeCl2 + Cl2 -> FeCl3}\]
Write your observation for the following chemical reaction and name the product formed :
When manganese dioxide is added to potassium chlorate and heated.
Write your observation for the following chemical reaction and name the product formed :
When water is added to quick lime?
Balance the following equation. Also name the product formed.
FeCI2 + CI2 → FeCI3
Complete the following equation:
CH4 + O2 —>
Complete the following equation:
CH3COOH + NaOH →
To determine the percentage of water absorbed by raisins, raisins are soaked in water for:
Balance the following equation:
MnO2 + HCl → MnCl2 + H2O + Cl2
Balance the following equation:
NaHCO3 → Na2CO3 + H2O + CO2
What information do the following chemical equations convey? Mg + 2HCl → MgCl2+ H2
Write the balanced chemical equations of the following reactions.
chlorine + sulphur dioxide + water → sulphuric acid + hydrogen chloride
Write the balanced chemical equation of the following reaction.
silver nitrate → silver + nitrogen dioxide + oxygen
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate mass of MnO2 used.
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate moles of acid required.
Write word equation for the following molecular equation:
CuCo3 → CuO + CO2 [g]
Word equation:
State the characteristics or indications occurring in the above reaction.
In certain reaction a change of state is observed i.e. solid to liquid, liquid to gas etc. – State the change of state of the products – to give the respective reactant.
NH3 + HCl ⇌ NH4Cl
Give word equation for the following chemical reaction and give the names of the product formed.
Zn + S → ZnS
Write word equation for the following chemical reaction given below. Also state the observation seen in the case.
\[\ce{ZnCo3->[\triangle] ZnO + CO2}\]
Give an example of a chemical equation in which two reactants form:
three product
Balance the following simple equation:
Al + H2SO4 → Al2(SO4)3 + H2
Write a balanced equation for the following word equation:
Potassium bromide + Chlorine → Potassium chloride + Bromine
With reference to a chemical equation state which of the statements 1 to 5 pertain to A or B.
A: Information provided by a chemical equation.
B: Limitations of a chemical equation.
- The nature of the individual elements.
- The speed of the reaction.
- The state of matter in which the substance is present.
- The completion of the reaction.
- The direction of the reaction.
Give a balanced equation by partial equation method, [steps are given below].
The reaction of excess ammonia with chlorine – Ammonia as a reducing agent
- Ammonia first reacts with chlorine to give hydrogen chloride and nitrogen.
- Hydrogen chloride then further reacts with excess ammonia to give ammonium chloride.
Write scientific reason.
Adding zinc particles to a solution of copper sulphate makes the blue solution colorless.
Complete the following blank in the equation as indicated.
\[\ce{CaH2_{(s)} + 2H2O_{( aq)}-> Ca(OH)2_{(s)} + 2H2_{(g)}}\]
Molecules: 6.02 × 1023 + ______ `→` ______ + ______
