Advertisements
Advertisements
प्रश्न
Write the equation for the reaction where the aluminum oxide for the electrolytic extraction of aluminum is obtained by heating aluminum hydroxide.
उत्तर

APPEARS IN
संबंधित प्रश्न
Aluminium is said to be more reactive than iron, towards oxygen (or air) yet iron undergoes corrosion to a greater extent than aluminum. Explain.
Name the constituents of Solder.
For sodium hydroxide, explain its significance in the extraction of aluminium.
In order to obtain 1 tonne of aluminium, the following inputs are required: 4 tonnes of bauxite, 150 kg of sodium hydroxide and 600 kg of graphite. The aluminium compound in bauxite is aluminium oxide and the main impurity is iron (III) oxide. Aluminium is obtained by the electrolysis of aluminium oxide dissolved in cryolite.
- Name the process used for the purification of bauxite.
- Write the equation for the action of heat on aluminium hydroxide.
Aluminium is extracted from its chief ore bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Name a chemicals used for dissolving aluminium oxide. In which state of sub-division is the chemical used?
The sketch below illustrates the refin ing of aluminium by Hoope's process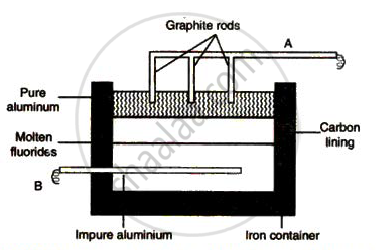
(a) Which of A and B is th e cathode and which one is the anode?
(b) What is the electroly te in the tank?
( c) What material is used for th e cathode?
The following question relate to the extraction of aluminium by electrolysis.
Name the other aluminium containing compound added to alumina and state the significance.
The following question relate to the extraction of aluminium by electrolysis.
Give the equation for the reaction that takes place at the cathode
Describe the role played in the extraction of aluminum : Graphite
Given below in column A is a schematic diagram of the electrolytic reduction of alumina. Identify the parts labelled as A, B and C with the correct options from the Column B.
| column A | column B | |
 |
1. | Platinum |
| 2. | Anode | |
| 3. | Cathode | |
| 4. | Electrolyte mixture | |
| 5. | Bauxite |
