Advertisements
Advertisements
Question
A solution of caustic soda (NaOH) in water or when fused, conducts an electric current. what is the similarity in these two cases?
Solution
Caustic soda (NaOH) in water or in a fused state conducts electricity due to the dielectric effect of the polar water molecules, which overcomes electrostatic attraction and allows the ions to move freely.
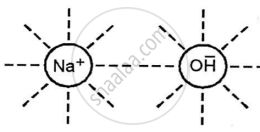
The high temperature needed to melt the solid weakens the bond between the particles in the molten state (fused), releasing the ions.
APPEARS IN
RELATED QUESTIONS
State the observations at the anode and at the cathode during the electrolysis of copper sulphate solution using copper electrodes.
Name the product formed at the anode during the electrolysis of acidified water using platinum electrodes.
Molten lead bromide conducts electricity. It is called an ______. It is composed of lead ______ and bromide ______. The lead ions are ______ charged and are called ______. The bromide ______ are ______ charged and are called ______.
Name a base which is a weak electrolyte.
Explain, why solid sodium chloride does not allow electricity to pass through?
Fill in the blank from the choices given below :
In covalent compounds, the bond is formed due to the ______ of electrons.
Give appropriate scientific reasons for the following statement :
Zinc oxide can be reduced to zinc by using carbon monoxide, but aluminium oxide cannot be reduced by a reducing agent.
Classify the following substance:
Carbon tetrachloride
Classify the following substance:
Sodium acetate
Classify the following substance:
Dilute sulphuric acid
