Advertisements
Advertisements
Question
Air pressure in a car tyre increases during driving. Explain.
Solution
During driving, the temperature of the gas inside the tyres increases due to the application of the reaction force on the tyres. But the volume of gas inside the tyre is constant. So, according to the Charles law P ∝ T, the air pressure of car tyres increases.
APPEARS IN
RELATED QUESTIONS
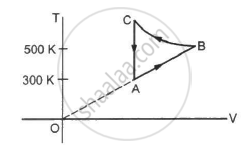
Consider the cyclic process ABCA, shown in figure, performed on a sample of 2.0 mol of an ideal gas. A total of 1200 J of heat is withdrawn from the sample in the process. Find the work done by the gas during the part BC.
Choose the correct option.
Which of the following is an example of the first law of thermodynamics?
The process, in which no heat enters or leaves the system, is termed as ____________.
Three copper blocks of masses M1, M2 and M3 kg respectively are brought into thermal contact till they reach equilibrium. Before contact, they were at T1, T2, T3 (T1 > T2 > T3). Assuming there is no heat loss to the surroundings, the equilibrium temprature T is (s is specific heat of copper)
Is it possible to increase the temperature of a gas without adding heat to it? Explain.
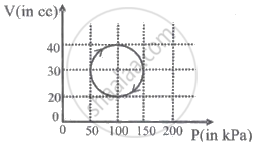
A system is taken through a cyclic process represented by a circle as shown. The heat absorbed by the system is ______.
ΔU = 0 is true for ______.
104 J of work is done on a certain volume of a gas. If the gas releases 125 kJ of heat, calculate the change in internal energy of the gas.
Write a short note on isobar.
What is an isothermal process?
