Advertisements
Advertisements
Question
What is an isothermal process?
Solution
A process in which change in pressure and volume takes place at a constant temperature is called an isothermal process or isothermal change. For such a system, ΔT = 0. An isothermal process is a constant temperature process.
APPEARS IN
RELATED QUESTIONS
An electric heater supplies heat to a system at a rate of 100W. If the system performs work at a rate of 75 Joules per second. At what rate is the internal energy increasing?
A system can be taken from the initial state p1, V1 to the final state p2, V2 by two different methods. Let ∆Q and ∆W represent the heat given to the system and the work done by the system. Which of the following must be the same in both the methods?
The internal energy of an ideal gas decreases by the same amount as the work done by the system.
(a) The process must be adiabatic.
b) The process must be isothermal.
(c) The process must be isobaric.
(d) The temperature must decrease.
Calculate the change in internal energy of a gas kept in a rigid container when 100 J of heat is supplied to it.
The pressure of a gas changes linearly with volume from 10 kPa, 200 cc to 50 kPa, 50 cc. (a) Calculate the work done by the gas. (b) If no heat is supplied or extracted from the gas, what is the change in the internal energy of the gas?
Choose the correct option.
Which of the following is an example of the first law of thermodynamics?
10 kg of four different gases (Cl2, CH4, O2, N2) expand isothermally and reversibly from 20 atm to 10 atm. The order of amount of work will be ____________.
A sample of gas absorbs 4000 kJ of heat and surrounding does 2000 J of work on sample. What is the value of ∆U?
The compressibility of water is 5 × 10-10 m2/N. Pressure of 15 × 106 Pa is applied on 100 ml volume of water. The change in the volume of water is ______.
"The mass and energy both are conserved in an isolated system", is the statement of ______.
The process, in which no heat enters or leaves the system, is termed as ____________.
In a given process for an ideal gas, dW = 0 and dQ < 0. Then for the gas ____________.
120 J of heat is added to a gaseous system, whose internal energy change is 60 J, then the amount of external work done is ____________.
Calculate the amount of work done during isothermal expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atmosphere?
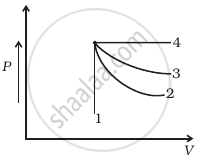
An ideal gas undergoes four different processes from the same initial state (figure). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic.
The initial state of a certain gas is (Pi, Vi, Ti). It undergoes expansion till its volume becomes Vf. Consider the following two cases:
- the expansion takes place at constant temperature.
- the expansion takes place at constant pressure.
Plot the P-V diagram for each case. In which of the two cases, is the work done by the gas more?
The first law of thermodynamics is concerned with the conservation of ______.
Write the mathematical equation for the first law of thermodynamics for:
Isothermal process
The amount of heat needed to raise the temperature of 4 moles of a rigid diatomic gas from 0°C to 50°C when no work is done is ______.
(R is the universal gas constant.)
In the reported figure, heat energy absorbed by a system in going through a cyclic process is ______ πJ.
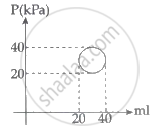
An ideal gas is taken through series of changes ABCA. The amount of work involved in the cycle is ______.
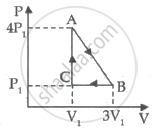
The first law of thermodynamics for isothermal process is ______.
If one mole of monoatomic gas `(gamma=5/3)` is mixed with one mole of diatomic gas `(gamma=7/5)`, the value of γ for the mixture is ______.
Derive an expression for the work done during an isothermal process.
One mole of an ideal gas is initially kept in a cylinder with a movable frictionless and massless piston at pressure of 1.01MPa, and temperature 27°C. It is then expanded till its volume is doubled. How much work is done if the expansion is isobaric?
If the adiabatic ratio for a gas is 5/3, find the molar specific heat capacity of the gas at (i) constant volume (ii) constant pressure.
Choose the correct relation with reason.
Obtain an expression for the workdone by a gas in an isothermal process.
Write a short note on isobar.
