Advertisements
Advertisements
Question
Derive an expression for the work done during an isothermal process.
Solution
Consider the isothermal expansion of an ideal gas. Let its initial volume be Vi and the final volumes be Vf. The work done in an infinitesimally small isothermal expansion is given by
dW = pdV
The total work done in bringing out the expansion from the initial volume Vi to the final volume Vf is given by,
W = `int_{V_i}^{V_f} pdV`
∴ W = `int_{V_i}^{V_f} (nRT)/V`dV .............`("for an ideal gas", p = "nRT"/V)`
∴ W = `nRTint_{V_i}^{V_f}(dV)/V`
∴ W = nRT(In Vf - In Vi)
∴ W = nRT In `V_f/V_i`

This is the required expression for the work done during an isothermal process.
APPEARS IN
RELATED QUESTIONS
The internal energy of an ideal gas decreases by the same amount as the work done by the system.
(a) The process must be adiabatic.
b) The process must be isothermal.
(c) The process must be isobaric.
(d) The temperature must decrease.
Calculate the heat absorbed by a system in going through the cyclic process shown in figure.
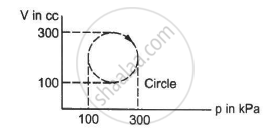
Choose the correct option.
Which of the following is an example of the first law of thermodynamics?
A solar cooker and a pressure cooker both are used to cook food. Treating them as thermodynamic systems, discuss the similarities and differences between them.
A resistor held in running water carries electric current. Treat the resistor as the system
- Does heat flow into the resistor?
- Is there a flow of heat into the water?
- Is any work done?
- Assuming the state of resistance to remain unchanged, apply the first law of thermodynamics to this process.
Which of the following are TRUE for a reversible isothermal process?
(i) ∆U = 0
(ii) ∆H = 0
(iii) Q = W
(iv) ∆T = 0
For a particular reaction, the system absorbs 8 kJ of heat and does 2.5 kJ of work on its surrounding. What will be the change in internal energy of the system?
Calculate the amount of work done during isothermal expansion of a gas from a volume of 4 dm3 to 6 dm3 against a constant external pressure of 3 atmosphere?
Change in internal energy, when 4 KJ of work is done on the system and 1 KJ heat is given out by the system, is:
If an average person jogs, hse produces 14.5 × 103 cal/min. This is removed by the evaporation of sweat. The amount of sweat evaporated per minute (assuming 1 kg requires 580 × 103 cal for evaparation) is ______.
Air pressure in a car tyre increases during driving. Explain.
Consider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical container is shown in figure.
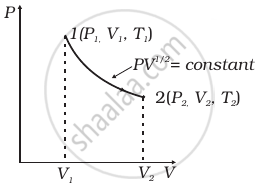
- Find the work done when the gas is taken from state 1 to state 2.
- What is the ratio of temperature T1/T2, if V2 = 2V1?
- Given the internal energy for one mole of gas at temperature T is (3/2) RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2 = 2V1.
A cycle followed by an engine (made of one mole of perfect gas in a cylinder with a piston) is shown in figure.
A to B : volume constant
B to C : adiabatic
C to D : volume constant
D to A : adiabatic
VC = VD = 2VA = 2VB
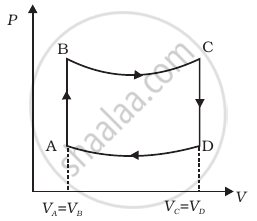
- In which part of the cycle heat is supplied to the engine from outside?
- In which part of the cycle heat is being given to the surrounding by the engine?
- What is the work done by the engine in one cycle? Write your answer in term of PA, PB, VA.
- What is the efficiency of the engine?
(γ = `5/3` for the gas), (Cv = `3/2` R for one mole)
Consider one mole of perfect gas in a cylinder of unit cross section with a piston attached (figure). A spring (spring constant k) is attached (unstretched length L) to the piston and to the bottom of the cylinder. Initially the spring is unstretched and the gas is in equilibrium. A certain amount of heat Q is supplied to the gas causing an increase of volume from V0 to V1.
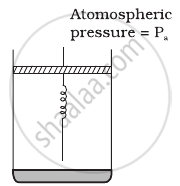
- What is the initial pressure of the system?
- What is the final pressure of the system?
- Using the first law of thermodynamics, write down a relation between Q, Pa, V, Vo and k.
The first law of thermodynamics is concerned with the conservation of ______.
200g water is heated from 40°C to 60°C. Ignoring the slight expansion of water, the change in its internal energy is close to ______.
(Given specific heat of water = 4184 J/kgK)
Mathematical equation of first law of thermodynamics for isochoric process is ______.
One mole of an ideal gas is allowed to expand reversibly and adiabatically from a temperature of 27°C. If the work done during the process is 3 kJ, the final temperature will be equal to ______.
(Cv = 20 JK−1)
Which among the following equations represents the first law of thermodynamics under isobaric conditions?
A soap bubble in vacuum has a radius of 3 cm and another soap bubble in vacuum has a radius of 4 cm. If the two bubbles coalesce under isothermal condition, then the radius of the new bubble is ______.
ΔU = 0 is true for ______.
The V cc volume of gas having `γ = 5/2` is suddenly compressed to `(V/4)` cc. The initial pressure of the gas is p. The final pressure of the gas will be ______.
An ideal gas (γ = 1.5) is expanded adiabatically. How many times has the gas had to be expanded to reduce the root mean square velocity of molecules two times?
In an adiabatic process, ______.
If the adiabatic ratio for a gas is 5/3, find the molar specific heat capacity of the gas at (i) constant volume (ii) constant pressure.
For an isothermal and reversible expansion of 0.5 mol of an ideal gas Wmax is - 3.918 kJ. The value of ΔU is ______.
Show that the heat absorbed at constant pressure is equal to the change in enthalpy of the system.
Write a short note on isobar.
