Advertisements
Advertisements
Question
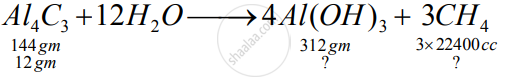
Aluminium carbide reacts with water according to the following equation :
`Al_4C_3 + 12H_2O-> 4Al(OH)_3 + 3CH_4`
1)What mass of aluminium hydroxide is formed from 12g of aluminium carbide?
2) What volume of methane at s.t.p. is obtained from 12g of aluminium carbide?
[Relatively molecular weight of `Al_4Cl_3 = 144; Al(OH)_3 = 78]`
Solution
1)
So, the amount of 3 Al(OH)3 formed will be 26 gm
2) From 12 gm Al4C3 5600 cc methane will be formed
APPEARS IN
RELATED QUESTIONS
Calculate the relative molecular mass of Sodium acetate
(use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S= 32)
Calculate the relative molecular mass of Chloroform.
(use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S= 32)
The usefulness of a fertilizer depends upon percentage of nitrogen present in it. Find which of the following is a better fertilizer:
(a) Ammonium nitrate [NH4NO3]
(b) Ammonium phosphate [(NH4)3PO4 (N=14,H=1,O=16,P=31)
An organic compound has the following percentage composition: C = 12.76%, H = 2.13%, Br = 85.11%. The vapour density of the compound is 94. Find out its molecular formula.
Concentrated nitric acid oxidizes phosphorous to phosphoric acid according to the following equation :
P + 5HNO3 → H3PO4 + H2O + 5NO2
What mass of phosphoric acid can be prepared from 6 .2 g of phosphorous?
Calculate the relative molecular mass of:
Potassium chlorate
Calculate the relative molecular mass of:
CHCl3
The atomic mass of Chlorine is 35.5. What is its vapour density?
A gas cylinder can hold 1 kg of hydrogen at room temperature and pressure. What mass of carbon dioxide can it hold under similar conditions of temperature and pressure?
If a crop of wheat removes 20 kg of nitrogen per hectare of soil, what mass of the fertilizer, calcium nitrate Ca(NO3)2 would be required to replace the nitrogen in a 10 hectare field?
