Advertisements
Advertisements
Question
Answer the following.
Name the polymer type in which following linkage is present.
\[\begin{array}{cc}\ce{- C - O -}\\||\phantom{.....}\\
\ce{O\phantom{.....}}\end{array}\]
Solution
The polymer containing ester linkage is called polyester.
APPEARS IN
RELATED QUESTIONS
Write any ‘two' uses of terylene.
Bakelite is the polymer of:
(a) Benzaldchyde and phenol
(b) Acetaldehyde and phenol
(c) Formaldehyde and phenol
(d) Formaldehyde and benzyl alcohol
Based on molecular forces, what type of polymer is neoprene?
Write the structure of melamine.
Write the structures of the monomers used for getting the following polymers
Melamine – formaldehyde polymer
Draw the structures of veronal and thymine.
Answer the following in one sentence.
Identify 'A' in the following reaction:
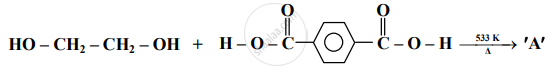
Answer the following in one sentence.
Identify 'B' in the following reaction:
\[\ce{H2N -(CH2)6 - NH2 + HOOC - (CH2)4 - COOH ->[N2][533 K]}\]'B'
Answer the following in one sentence.
What type of intermolecular force leads to high-density polymer?
Identify condensation polymers and addition polymers from the following.
-(CH2 - CH = CH - CH2 -)n
Identify condensation polymers and addition polymers from the following.
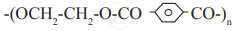
Write the structure of isoprene and the polymer obtained from it.
Monomer used for preparation of polyacrylonitrile is _____________
Write chemical reaction for preparation of the following.
Buna-S
Write chemical reaction for preparation of the following.
Neoprene
Which among the following polymers is obtained from styrene and 1-3-butadiene?
Which among the following polymers is used for making handles of cooker?
Identify additional polymers from the following.
I. \[\begin{array}{cc}
\ce{-(CH2 - CH -)_{{n}}}\\
\phantom{....}|\\
\phantom{.......}\ce{C6H5}
\end{array}\]
II. \[\ce{-(CH2 - CH = CH - CH2 -)_{{n}}}\]
III. \[\ce{-(CO(CH2)4 - CONH(CH2)6NH -)_{{n}}}\]
IV.
![]()
Identify the CORRECT statement regarding the following polymer.
\[\begin{array}{cc}
\phantom{....}\ce{O}\phantom{............}\ce{O}\phantom{...................}\ce{H}\phantom{.....}\\
\phantom{....}||\phantom{.............}||\phantom{...................}|\phantom{......}\\
\ce{-[C - (CH2)4 - C - NH - (CH2)6 - N -]_{{n}}}
\end{array}\]
The INCORRECT match for the polymer with its application is:
Identify the INCORRECT match.
Which of the following is the monomer of neoprene?
Select the CORRECT match for both the polymers.
Which of the following polymers is a heteropolymer?
Identify the polymer obtained by polymerization of n moles of acrylonitrile.
Which among the following polymers can NOT be remoulded?
Which of the following compounds is used to prepare orlon?
Which among the following polymers is obtained from CH2 = CH – CN by polymerisation?
Which of the following polymers is prepared by using phenol?
Which of the following polymer is used to make blankets?
Identify addition polymer from the following.
Name the polymers used in laminated sheets and give the name of monomeric units involved in its formation.
Match the polymers given in Column I with the type of linkage present in them given in Column II.
| Column I | Column II |
| (i) Terylene | (a) Glycosidic linkage |
| (ii) Nylon | (b) Ester linkage |
| (iii) Cellulose | (c) Phosphodiester linkage |
| (iv) Protein | (d) Amide linkage |
| v) RNA |
Phenol and formaldehyde undergo condensation to give a polymar (A) which on heating with formaldehyde gives a thermosetting polymer (B). Name the polymers. Write the reactions involved in the formation of (A). What is the structural difference between two polymers?
Which of the following polymer has ester linkage?
Which one of the following polymers are prepared by addition polymerization?
Which among the following polymers has high tensile strength and is used to obtain tyre cords?
Which of the following is a polymer of enzyme?
Answer the following.
Write the structure of isoprene and the polymer obtained from it.
Name and draw the structure of the repeating unit in natural rubber.
How the Bakelite is prepared? Give the steps involved in the preparation.
Another name of terylene is ______.
Match the following pairs:
| Polymer | Monomer | ||
| (i) | Teflon | (a) | CH2 = CH2 |
| (ii) | PVC | (b) | CF2 = CF2 |
| (iii) | Natural rubber | (c) | Isoprene |
| (iv) | Polythene | (d) | H2C=CHCl |
Name and draw the structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
