Advertisements
Advertisements
Question
Answer the following
Write a reaction to convert Aniline into p-bromoaniline.
Solution
–NH2 group in aniline is highly ringing activating and o-/p- directing due to the involvement of the lone pair of electrons on ‘N’ in resonance with the ring. As a result, on reaction with Br2, it gives 2,4,6-tribromoniline. To get a monobromo product, it is necessary to decrease the ring activating effect of -NH2 group. This is done by acetylation of aniline. The lone pair of ‘N’ in acetanilide is also involved in resonance in the acetyl group. To that extent ring activation decreases.

Hence, acetanilide on bromination gives a monobromo product p-bromoacetanilide. After monobromination, the original -NH2 group is regenerated. The protection of the -NH2 group in the form of acetyl group is removed by acid-catalyzed hydrolysis to get p-bromoaniline, as shown in the following scheme.

APPEARS IN
RELATED QUESTIONS
Answer the following
Complete the following reaction :
\[\ce{C6H5NH2 + Br2_{(aq)} -> ?}\]
Answer the following.
Write a reaction to bring about the following conversions: Aniline into p-nitroaniline
Answer the following.
Write a reaction to bring about the following conversions: Aniline into sulphanilic acid?
Aniline on reaction with bromine water produces ________________
The following compound is a/an ____________.
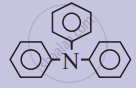
Which of the following molecules form a Zwftter ion?
Which of the following molecule can form a zwitter ion?
Identify the CORRECT statements.
(I) Aniline reacts with bromine water at room temperature to give o-bromoaniline.
(II) Aniline undergoes Friedel Craft's reaction using aluminium chloride.
(III) Direct nitration of aniline yields, a mixture of ortho, meta and para nitro aniline.
(IV) Amino group is ortho and para directing and powerful ring activating group.
Identify the product obtained when chlorobenzene is heated with ammonia and Cu2O at 473 K under pressure.
Which of the following groups increases the basic strength of substituted aniline?
The major product obtained when aniline undergoes sulphonation using cone. sulphuric acid at 453-473 K is:
Aniline on reaction with Bromine water at room temperature gives ____________.
When propanamide is treated with bromine and aqueous sodium hydroxide, the compound formed is ____________.
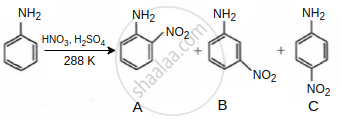
Consider the given reaction, percentage yield of:
In case of substituted aniline the group which decreases the basic strength is ______.
What is the action of aqueous bromine on aniline?
