Advertisements
Advertisements
Question
Atomic number of \[\ce{Mn}\], \[\ce{Fe}\] and \[\ce{Co}\] are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complex ions are diamagnetic?
(i) \[\ce{[Co(NH3)6]^{3+}}\]
(ii) \[\ce{[Mn(CN)6]^{3-}}\]
(iii) \[\ce{[Fe(CN)6]^{4-}}\]
(iv) \[\ce{[Fe(CN)6]^{3-}}\]
Solution
(i) \[\ce{[Co(NH3)6]^{3+}}\]
(iii) \[\ce{[Fe(CN)6]^{4-}}\]
Explanation:
(i) Molecular orbital electronic configuration of \[\ce{Co^{3+}}\] in \[\ce{[Co(NH3)6]^{3+}}\] is
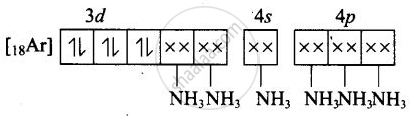
Number of unpaired electron = 0
Magnetic property = Diamagnetic
(ii) Molecular orbital electronic configuration of \[\ce{Mn^{3+}}\] in \[\ce{[Mn(CN)6]^{3-}}\]
Number of unpaired electron = 2
Magnetic property = Paramagnetic
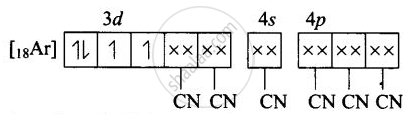
(iii) Molecular orbital electronic configuration of \[\ce{Fe^{3+}}\] in \[\ce{[Fe(CN)6]^{4-}}\] is
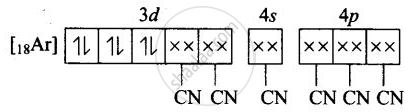
Number of unpaired electron = 0
Magnetic property = Diamagnetic
(iv) Molecular orbital electronic configuration of \[\ce{Fe^{3+}}\] in \[\ce{[Fe(CN)6]^{3-}}\]
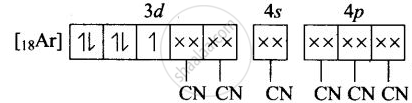
Number of unpaired electron = 1
Magnetic property = Paramagnetic
APPEARS IN
RELATED QUESTIONS
How does the magnitude of Δ0 decide the actual configuration of d orbitals in a coordination entity?
The colour of the coordination compounds depends on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, \[\ce{[Co(NH3)6]^{3+}}\], \[\ce{[Co(CN)6]^{3-}}\], \[\ce{[Co(H2O)6]^{3+}}\]
Give the electronic configuration of the following complexes on the basis of Crystal Field Splitting theory.
\[\ce{[CoF6]^{3-}, [Fe(CN)6]^{4-} and [Cu(NH3)6]^{2+}}\].
\[\ce{CuSO4 . 5H2O}\] is blue in colour while \[\ce{CuSO4}\] is colourless. Why?
Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following:
\[\ce{[FeF6]^{3-}, [Fe(H2O)6]^{2+}, [Fe(CN)6]^{4-}}\]
The correct order of increasing crystal field strength in following series:
For octahedral Mn(II) and tetrahedral Ni(II) complexes, consider the following statements:
(i) Both the complexes can be high spin.
(ii) Ni(II) complex can very rarely below spin.
(iii) With strong field Ligands, Mn(II) complexes can be low spin.
(iv) Aqueous solution of Mn (II) ions is yellow in colour.
The correct statements are:
The complex that has highest crystal field splitting energy (Δ) is ______.
On the basis of crystal field theory, write the electronic configuration for the d5 ion with a weak ligand for which Δ0 < P.
Read the passage carefully and answer the questions that follow.
|
Crystal field splitting by various ligands Metal complexes show different colours due to d-d transitions. The complex absorbs light of specific wavelength to promote the electron from t2g to eg level. The colour of the complex is due to the transmitted light, which is complementary of the colour absorbed. The wave number of light absorbed by different complexes of Cr ion are given below:
|
Answer the following questions:
(a) Out of ligands "A", "B", "C" and "D", which ligand causes maximum crystal field splitting? Why?
OR
Which of the two, “A” or “D” will be a weak field ligand? Why?
(b) Which of the complexes will be violet in colour? [CrA6]3- or [CrB6]3+ and why?
(Given: If 560 - 570 nm of light is absorbed, the colour of the complex observed is violet.)
(c) If the ligands attached to Cr3+ ion in the complexes given in the table above are water, cyanide ion, chloride ion, and ammonia (not in this order).
Identify the ligand, write the formula and IUPAC name of the following:
- [CrA6]3-
- [CrC6]3+
